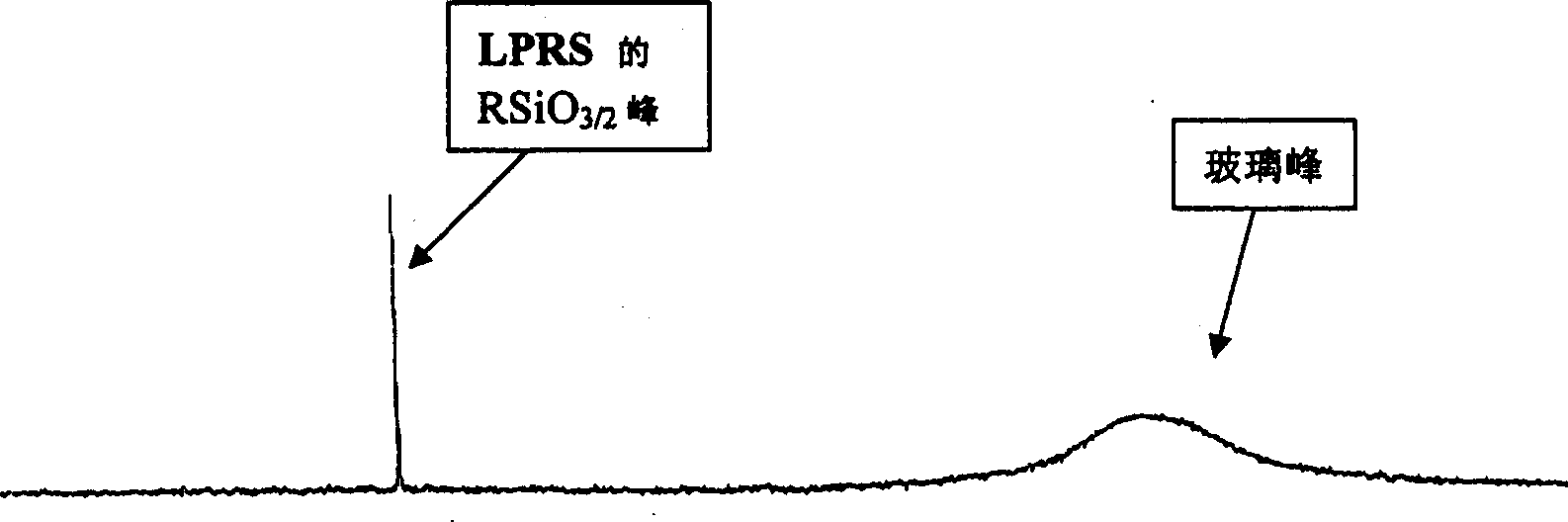
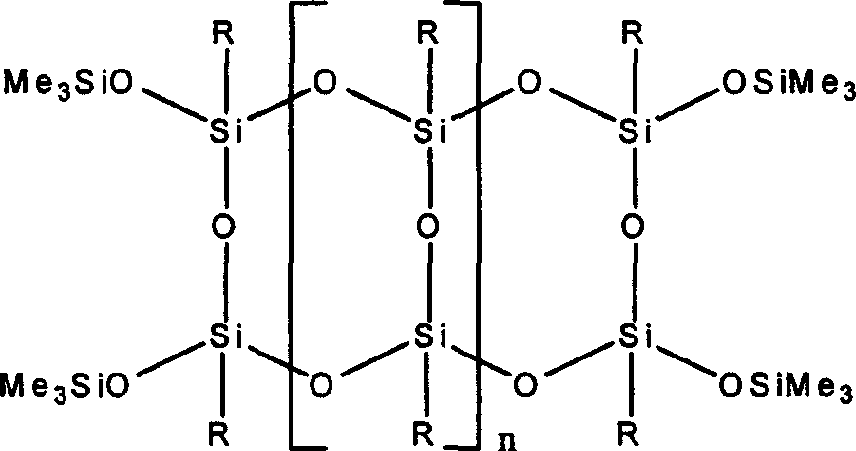
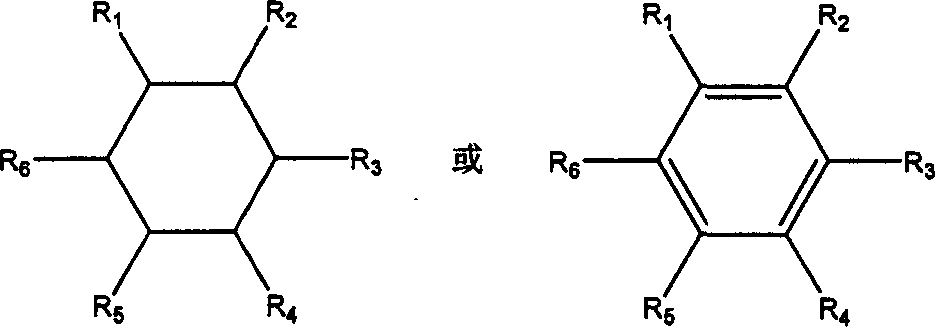
Trapezoidal organic poly sesquisiloxane and preparation method
A silsesquioxane and organic technology, applied in the field of preparation of the above-mentioned ladder polyorganosilsesquioxane, can solve the problems of preparation, lack of verification, lack of mature methods and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] With stirring, a 50ml dioxane solution containing 1.08g (10mmol) PDA was added dropwise to a solution containing 4.3g (20mmol) phenyltrichlorosilane (PhSiCl 3 ) and 6.1g (60mmol) of triethylamine (TEA) in 50ml of chlorobenzene solution, the temperature was maintained at 0°C, after stirring for half an hour, 10ml of dioxane solution containing 0.36ml (20mmol) of water was added dropwise, and stirred at room temperature for 3 Filter after 1 hour, its filtrate removes most of the solvent with a rotary evaporator, adds anhydrous sodium sulfate to dry, takes out the upper layer drying solution, adds a capping agent trimethylchlorosilane 10.8mg (0.10mmol) to end capping, then adds 0.13g of water and and Stir 1-5 drops of concentrated sulfuric acid at 40 degrees for 24 hours, then wash with saturated aqueous sodium chloride until neutral, and dry with anhydrous sodium sulfate to obtain a colorless and transparent toluene solution of trapezoidal polyphenylsilsesquioxane. 15mg / m...
Embodiment 2
[0098] With stirring, a solution of 25ml of dioxane containing 1.08g (10mmol) of PDA was added dropwise to a solution containing 3.0g (20mmol) of methyltrichlorosilane (MeSiCl 3 ) and 6.1g (60mmol) triethylamine (TEA) in 40ml of toluene solution, the temperature is controlled at about -15°C, stirred for half an hour, and then 20ml dioxane solution containing 0.36ml (20mmol) water is added dropwise to the reaction bottle, stirred at room temperature for 4 hours and filtered, and the filtrate was removed with a rotary evaporator to remove most of the solvent, dried with anhydrous sodium sulfate, filtered to obtain the filtrate and added 10.8 mg (0.10 mmol) of capping agent trimethylchlorosilane under stirring. Then add 0.23g of water and 1-5 drops of concentrated sulfuric acid, stir at room temperature for three hours, raise the temperature to 80-90°C and stir for 5 hours, then wash with saturated aqueous sodium chloride until neutral, and dry with anhydrous sodium sulfate to obt...
Embodiment 3
[0100] Under stirring, 50ml of dioxane solution containing 2.16g (20mmol) pre-aminolysis agent PDA was added dropwise to 6.5g (40mmol) vinyl trichlorosilane (CH 2 =CHSiCl 3 ) and 12.2g (120mmol) of triethylamine (TEA) in 80ml of chlorobenzene solution, the temperature was maintained at about -20°C, after the dropwise addition was completed, the stirring was continued for half an hour, and then the 10ml of tetrahydrofuran solution containing 0.72g (40mmol) of water was slowly Add it dropwise into the reaction flask, stir for 4 hours, filter, remove 1 / 2 of the solvent with a rotary evaporator for the filtrate, and wash the remaining chlorobenzene solution with anhydrous Na 2 SO 4 Dry, take the supernatant liquid with 21.8mg (0.20mmol) trimethylsilyl chloride (Me 3 SiCl) head, add 0.6g of water and 1-5 drops of concentrated sulfuric acid for dehydration condensation, stir at room temperature for 24 hours, wash with saturated aqueous sodium chloride solution until neutral, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com