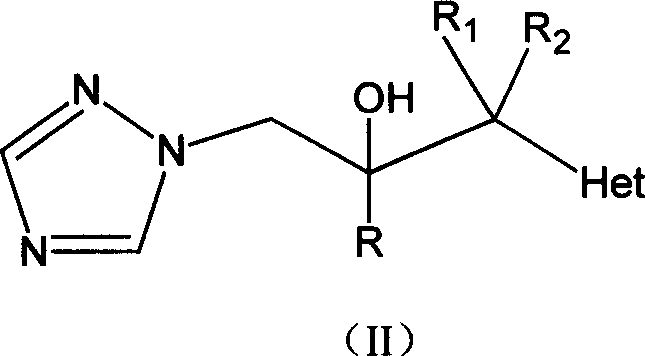
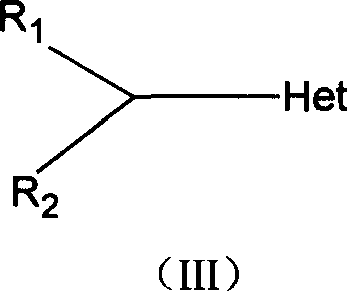
Prepn process and intermediate for voriconazole
A technology for triazoles and compounds, applied in the field of compounds and their preparation, can solve the problems of difficult separation, low enantiomeric yield, unsuitable for large-scale economical production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
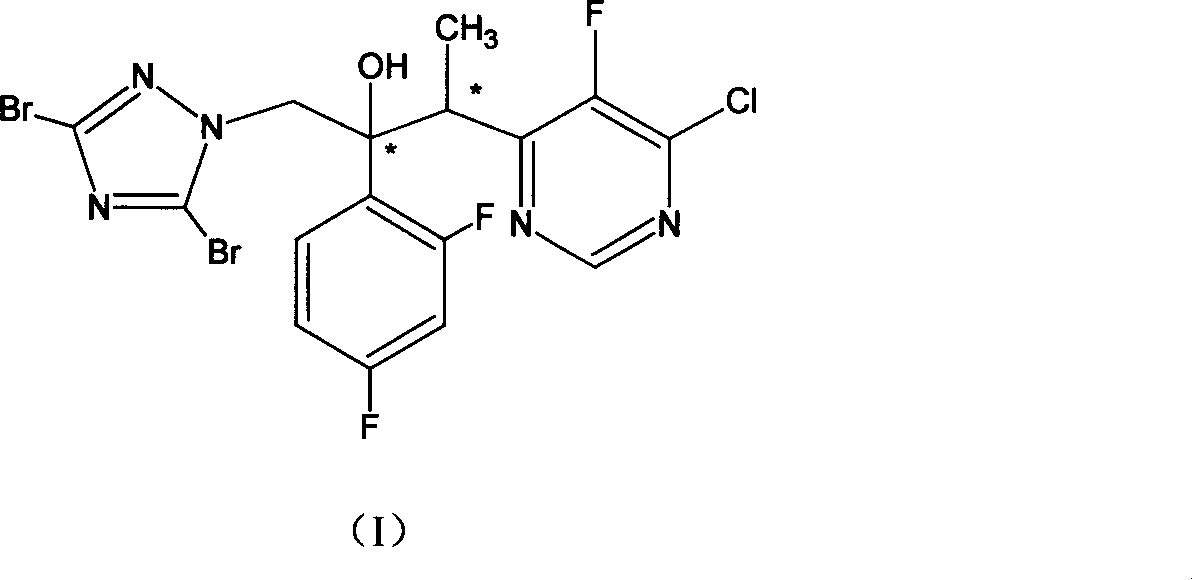
[0049] 1. Synthesis of 1-(2,4-difluorophenyl)-2-(3,5-dibromo-1H-[1,2,4]triazol-1-yl)ethanone
[0050]In a 500ml three-necked flask, add 2'-chloro-2,4-difluoroacetophenone (27.3g, 0.14mol), 3,5-dibromo-1H-[1,2,4]triazole ( 34.0g, 0.15mol), potassium carbonate (27.6g, 0.2mol), tetrabutylammonium bromide (2.0g) and dichloromethane (300ml), stirred at reflux for 8h. Cool, filter, filter cake with CH 2 Cl 2 Wash, combine the organic layers, wash with water, dry, concentrate to dryness, and recrystallize with petroleum ether / ethyl acetate (1 / 1, 200ml) to obtain 35.0g of light yellow solid with a yield of 65.6%.
[0051] 2. (2R, 3S / 2S, 3R)-3-(4-chloro-5-fluoropyrimidin-6-yl)-2-(2,4-difluorophenyl)-1-(3,5-di Synthesis of bromo-1H-[1,2,4]-triazol-1-yl)butan-2-ol
[0052] Under nitrogen, 2.5M hexane solution of n-butyl lithium (80ml, 0.2mol) was added in the three-necked flask, and a tetrahydrofuran (100ml) solution of diisopropylamine (20.0g, 0.2mol) was added dropwise under an ice...
Embodiment 2
[0058] 1. (2R, 3S / 2S, 3R)-3-(4-chloro-5-fluoropyrimidin-6-yl)-2-(2,4-difluorophenyl)-1-(3,5-di Preparation of bromo-1H-[1,2,4]-triazol-1-yl)butan-2-ol
[0059] Under nitrogen, 2.5M hexane solution of n-butyl lithium (80ml, 0.2mol) was added in the three-necked flask, and a tetrahydrofuran (100ml) solution of diisopropylamine (20.0g, 0.2mol) was added dropwise under an ice-water bath, Stir for 30 min, cool to -78°C, add dropwise 4-chloro-5-fluoro-6-ethylpyrimidine (32.1 g, 0.2 mol) in tetrahydrofuran (300 ml), and stir at this temperature for 3 h. Add 1-(2,4-difluorophenyl)-2-(3,5-dibromo-1H-[1,2,4]triazol-1-yl)ethanone (76.2 g, 0.2 mol) THF (500ml) solution, stirred at -78°C for 2h, slowly warmed to -20°C, continued to stir for 2h, cooled to -50°C, added a solution of glacial acetic acid (12.0g) in water (100ml) to stop the reaction, and heated to At room temperature, separate the organic layer, extract the aqueous layer with ethyl acetate (50ml×2), combine the organic layer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com