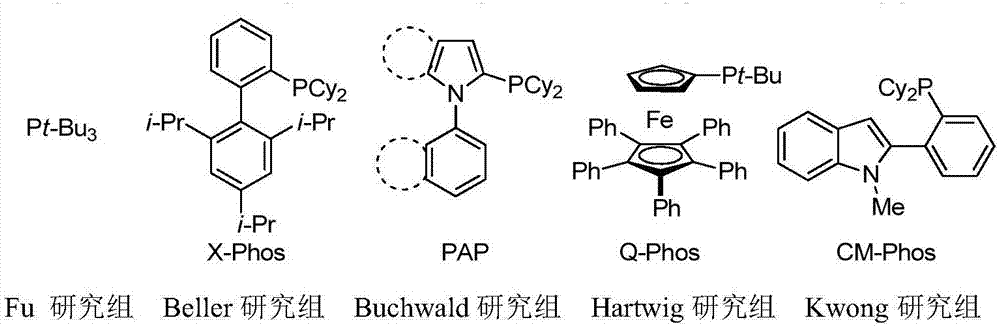
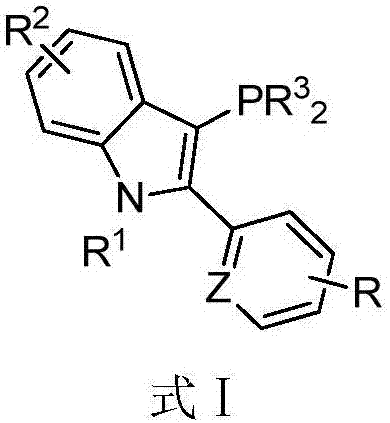
Phosphine ligand for indole skeleton as well as preparation method and application of phosphine ligand
A phosphine ligand, indole phosphine technology, applied in the field of organic compounds and synthesis, can solve problems such as poor catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] As a specific embodiment, a method for preparing a phosphine ligand of a 3-(disubstituted phosphino)-1-alkyl-2-substituted phenyl-indole skeleton is provided, comprising the following steps:
[0057] S01. After mixing substituted acetophenone and N-alkylphenylhydrazine, adding phosphoric acid as a catalyst, after stirring, adding polyphosphoric acid, and performing a heating reaction to prepare 2-(substituted phenyl)-1-alkane Base-1H-indole intermediate, or the substituted acetophenone and N-alkylphenylhydrazine are mixed, then add acetic acid and ethanol to react at 70-80°C for 0.5-1.5 hours, and then remove all solvents under reduced pressure , then add polyphosphoric acid, carry out heating reaction, prepare 2-(substituted phenyl)-1-alkyl-1H-indole intermediate, wherein, the heating temperature of described heating reaction is 80-120 ℃, and reaction time is 1 -2 hours;
[0058] S02. Dissolve the 2-(substituted phenyl)-1-alkyl-1H-indole intermediate and N-bromosuccin...
Embodiment 1
[0180] Example 1: Synthesis of 3-(dicyclohexylphosphino)-1-methyl-2-phenyl-1H-indole
[0181] In a 250 ml round bottom flask, add 10.4 g of N-methyl-2-phenylindole (50 mmol), then add 80 ml of anhydrous dimethylformamide and stir evenly. Then, a mixed solution of 10.6 g of N-bromosuccinimide (60 mmol) and 60 ml of anhydrous dimethylformamide was added and reacted at room temperature for 2 hours. When the reaction was completed, the reaction mixture was poured into ice water, and then 100 ml of dichloromethane and 50 ml of water were added. Then add 150 milliliters of water to wash each organic phase in five times again, and combine the organic phases. After all the solution was sucked off under reduced pressure, the concentrated reaction mixture was purified by column chromatography to obtain 12.3 grams of white powdery pure product 3-bromo-1-methyl-2-phenyl-1H-indole intermediate, the yield 86%. 1H NMR (400MHz, CDCl 3 ) 3.76 (s, 3H), 7.50-7.56 (m, 3H), 7.65-7.72 (m, 5H), ...
Embodiment 2
[0183] Example 2: Synthesis of 3-(dicyclohexylphosphino)-2-(2-methoxyphenyl)-1-methyl-1H-indole
[0184] In a 100 ml round bottom flask, add 6.9 ml of 2'-methoxyacetophenone (50 mmol) and 7.1 ml of N-methylphenylhydrazine (60 mmol), then slowly add 10 ml of phosphoric acid, and stir evenly at room temperature for 0.5 Hour. Then slowly add 50 grams of polyphosphoric acid. With the progress of the reaction, the system exothermic obviously. Then the mixture was slowly heated to 120°C and kept at 120°C for 1 hour. The mixture was poured into ice water to terminate the reaction, then 200 ml of ether was added to the system, and 200 ml of ether was added three times for extraction. The organic phases were combined and dried over anhydrous sodium sulfate. After all the solution was sucked off under reduced pressure, the concentrated reaction mixture was purified by column chromatography, and then the product was obtained as light yellow powder. Then add 15 milliliters of normal h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com