Tetrandrine solid lipide nano particle and its preparing method
A technology of solid lipid nanometer and tetrandrine, which is applied to the solid lipid nanoparticle preparation of tetragonin and its preparation, and the field of active ingredients of traditional Chinese medicine fangji, which can solve the problem of large toxic and side effects, small safety range, and application range. Restrictions and other issues, to achieve the effects of formulation optimization, high encapsulation efficiency, and small particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Weigh 70 mg of tetrandrine, 60 mg of stearic acid and 150 mg of soybean lecithin, and dissolve them in 10 ml of absolute ethanol in a 70°C water bath to form an organic phase solution.
[0030] (2) Measure 15ml of glycerin and disperse in 30ml of distilled water to form a water phase, and place it on a constant temperature magnetic stirrer at a temperature of 80°C and a stirring speed of 1000rpm.
[0031] (3) Inject the organic phase obtained in step (1) into the aqueous phase obtained in step (2), and stir for 1.5 hours to form a milky white suspension and form colostrum.
[0032] (4) ultrasonically treat the colostrum prepared in step (3) for 300 seconds, stir to room temperature, filter with a 0.45 μm microporous membrane to remove the titanium particle impurities released by the ultrasonic probe, and obtain tetrandrine solid lipid nanoparticle water Dispersion, sealed and stored at 4°C.
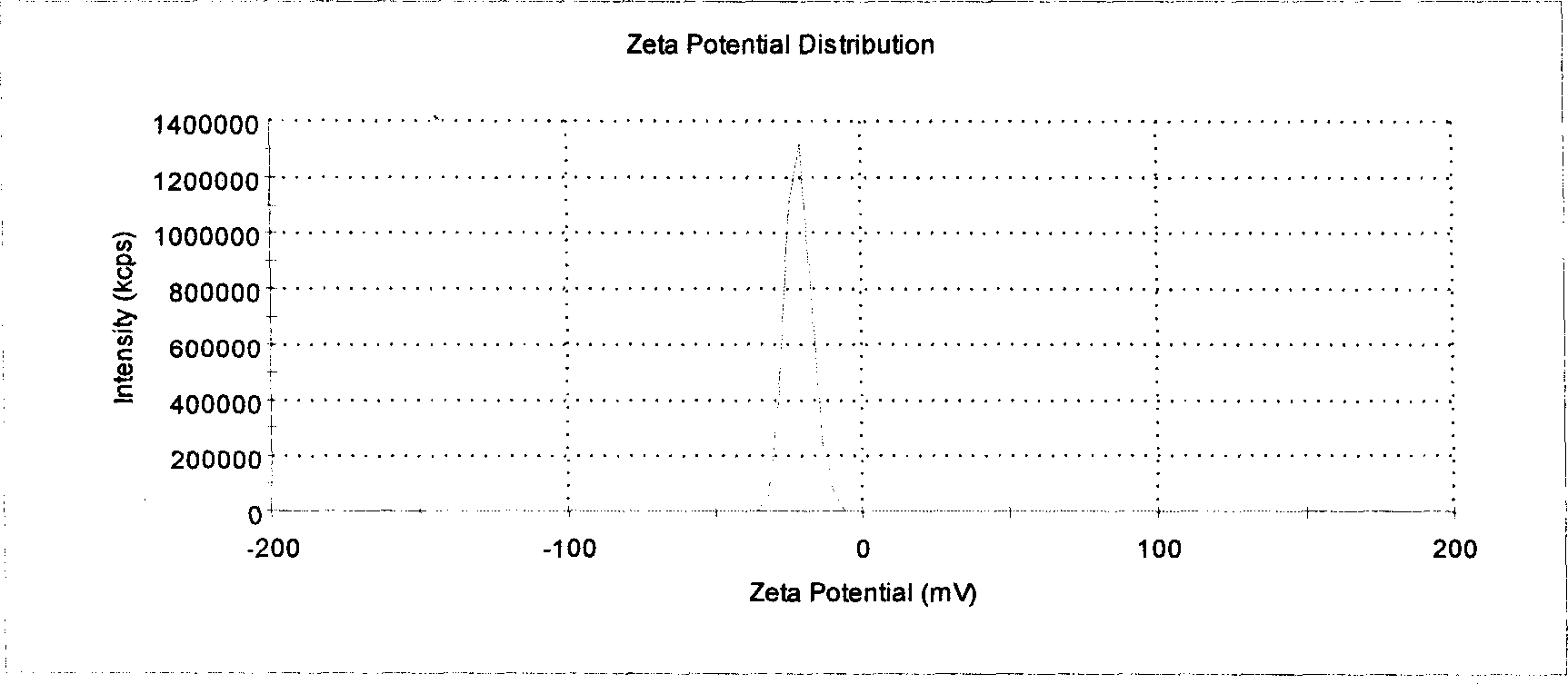
[0033] Detection: the average particle diameter of the tetrandrine solid ...
Embodiment 2
[0035] (1) Weigh 65 mg of tetrandrine, 45 mg of cholesterol and 120 mg of lecithin, and dissolve them in 9.5 ml of ethyl acetate in a water bath at 70°C to form an organic phase solution.
[0036] (2) Measure 15ml of mannitol and disperse in 30ml of distilled water to form an aqueous phase, and place it on a constant temperature magnetic stirrer at a temperature of 75°C and a stirring speed of 800rpm.
[0037] (3) Inject the organic phase prepared in step (1) into the aqueous phase prepared in step (2), and stir for 1 hour to form a milky white suspension and form colostrum.
[0038](4) ultrasonically treat the colostrum prepared in step (3) for 400 seconds, stir to room temperature, filter with a 0.45 μm microporous membrane to remove impurities such as titanium particles released by the ultrasonic probe, and obtain tetrandrine solid lipid nanoparticles Water dispersion, sealed and stored at 4°C.
[0039] Detection: The Tetrandrine solid lipid nanoparticles prepared by this ...
Embodiment 3
[0041] (1) Weigh 250 mg of tetrandrine, 150 mg of stearic acid and 450 mg of soybean lecithin, and dissolve them in 30 ml of absolute ethanol in a 75°C water bath to form an organic phase solution.
[0042] (2) Measure 45ml of glycerin and disperse in 95ml of distilled water to form a water phase, and place it on a constant temperature magnetic stirrer at a temperature of 80°C and a stirring speed of 1300rpm.
[0043] (3) Inject the organic phase prepared in step (1) into the aqueous phase prepared in step (2), and stir for 2 hours to form a milky white suspension and form colostrum.
[0044] (4) ultrasonically treat the colostrum prepared in step (3) for 600 seconds, stir to room temperature, filter with a 0.45 μm microporous membrane to remove impurities such as titanium particles released by the ultrasonic probe, and obtain tetrandrine solid lipid nanoparticles Water dispersion, sealed and stored at 4°C.
[0045] Detection: The Tetrandrine solid lipid nanoparticles prepare...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com