Compound medicinal formulation for treating acute-chronic vespiratory tract infection and its preparing method
A technology for pharmaceutical preparations and respiratory tract, applied in the field of medicine, can solve the problems of affecting the therapeutic effect, large dosage, difficult dosage control, etc., and achieve the effects of improving the therapeutic effect, taking a small dosage and facilitating clinical application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
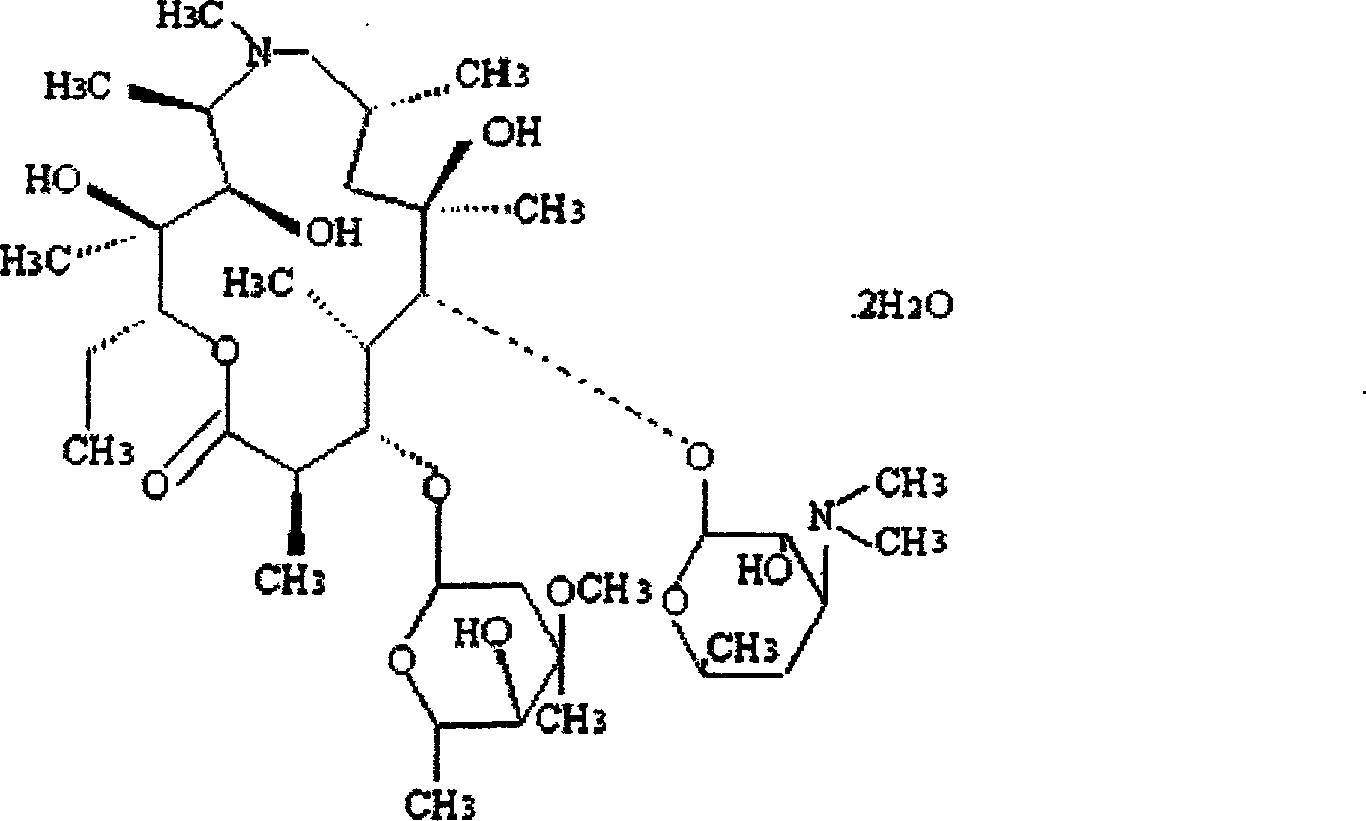
[0029] Embodiment 1: the preparation of azithromycin ambroxol sheet
[0030] Prescription: Azithromycin hydrochloride 250g (calculated as azithromycin)
[0031] Ambroxol Hydrochloride 60g
[0032] Starch 30g
[0033] Microcrystalline Cellulose 45g
[0034] Micronized silica gel 2g
[0036] Magnesium Stearate 2.5g
[0037] Proper amount of hydroxypropyl methylcellulose (E-30) (4% solution)
[0038] Made into 1000 pieces
[0039] Preparation method: Prepare 4% hydroxypropyl methylcellulose (E-30) solution and set aside. Weigh 10 g of starch and dry at 105°C for 5 hours for later use. Take by weighing 20g of starch and azithromycin hydrochloride, ambroxol hydrochloride, microcrystalline cellulose of prescription quantity, mix, pulverize and cross 80 mesh sieves. Use 4% hydroxypropyl methylcellulose (E-30) solution to make the material into a soft material, granulate it with a 20-me...
Embodiment 2
[0039] Preparation method: Prepare 4% hydroxypropyl methylcellulose (E-30) solution and set aside. Weigh 10 g of starch and dry at 105°C for 5 hours for later use. Take by weighing 20g of starch and azithromycin hydrochloride, ambroxol hydrochloride, microcrystalline cellulose of prescription quantity, mix, pulverize and cross 80 mesh sieves. Use 4% hydroxypropyl methylcellulose (E-30) solution to make the material into a soft material, granulate it with a 20-mesh sieve, and dry it at 50-60°C until the water content in the granule is about 3%. Pass through a 20-mesh sieve for granulation, add dry starch (dried at 105°C for 5 hours), magnesium stearate, micronized silica gel, and talcum powder in the prescribed amount, and finally mix, measure the content of intermediates, and determine the tablet weight; tablet. Embodiment 2: the preparation of azithromycin fudosteine sheet
[0040] prescription:
[0041] Azithromycin hydrochloride 125g (calculated as azithromycin)
...
Embodiment 3
[0051] Embodiment 3: the preparation of azithromycin ambroxol dry suspension
[0052] prescription:
[0053] Azithromycin hydrochloride 250g (calculated as azithromycin)
[0054] Ambroxol Hydrochloride 30g
[0055] Sucrose 720g
[0056] Made into 1000g
[0057] Preparation method: Weigh azithromycin hydrochloride, ambroxol hydrochloride and sucrose according to the above prescription, crush them through a 60-mesh sieve, mix them evenly, and pack them in packaging bags, 1g per bag.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com