Visual colorimetric fluorine ion sensing test-paper and use thereof
A technology of fluoride ion and test paper, which is applied in the direction of analyzing materials through chemical reactions and observing the influence of chemical indicators on materials. It can solve problems such as difficult detection and achieve simple discrimination and good selective recognition. , Ease of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 (synthesis of coordination compound, improved synthetic method of the present invention)
[0030] 0.238 g (1.2 mmol) of 2,4-dinitrophenylhydrazine, bis(bipyridyl)(4,5-diazafluorenone) ruthenium complex 0.88 g (1 mmol) was added to a 5 ml A mixed solution of phosphoric acid in 25 ml of acetonitrile and 25 ml of ethanol was refluxed for 8 hours in a nitrogen atmosphere, concentrated to 5 ml, and 100 ml of ethanol solution was added to precipitate a brown precipitate, which was filtered and washed three times with ethanol. The precipitate was dissolved in 5 ml of water, and KPF was added 6 A dark black precipitate precipitated out, was filtered off, washed with ethanol and dried in vacuo, with a yield of 60%. Elemental analysis (C 10 h 8 N 2 ) 2 Ru(C 17 h 10 N 6 o 5 )(PF 6 ) 2 Values are C, 41.6; H, 2.6; N, 13.2%, theoretical values are C, 41.7; H, 2.4; N, 13.1%. NMR analysis 1 H NMR (500MHz, CD 3 CN, T-toluene sulfonic acid), δ (ppm): 8.96 (...
Embodiment 2
[0031] Embodiment 2 (selective experiment)
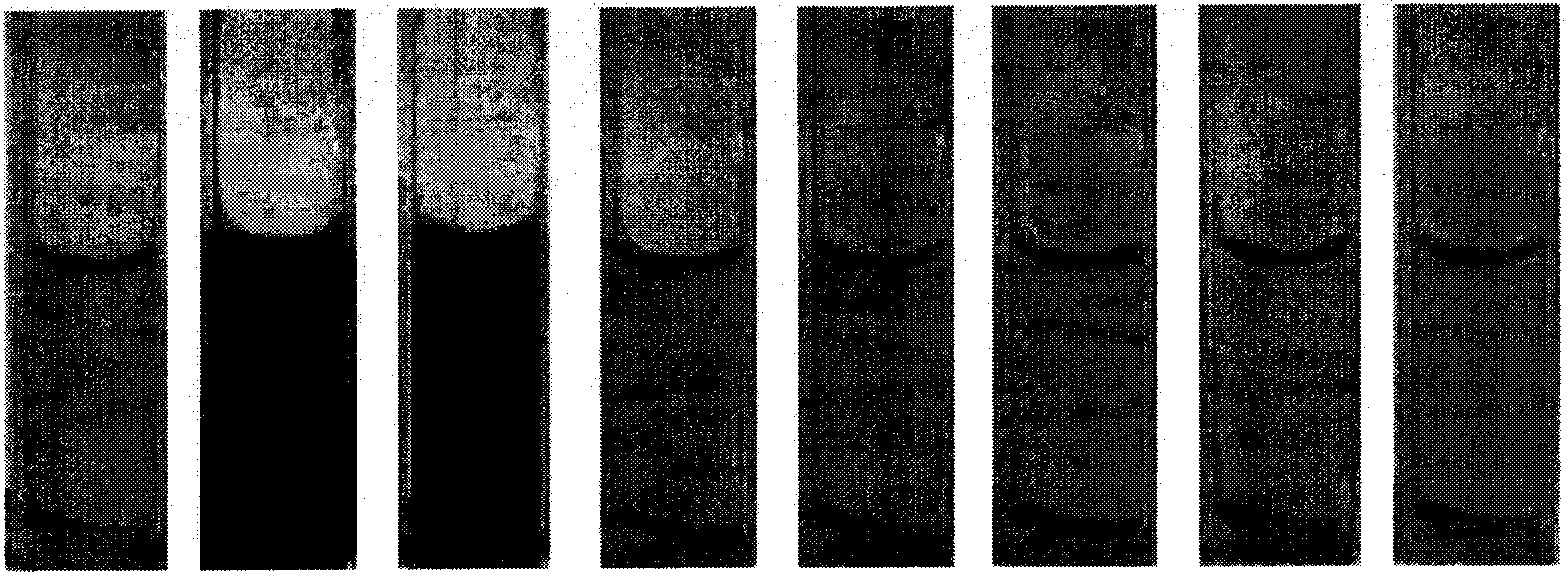
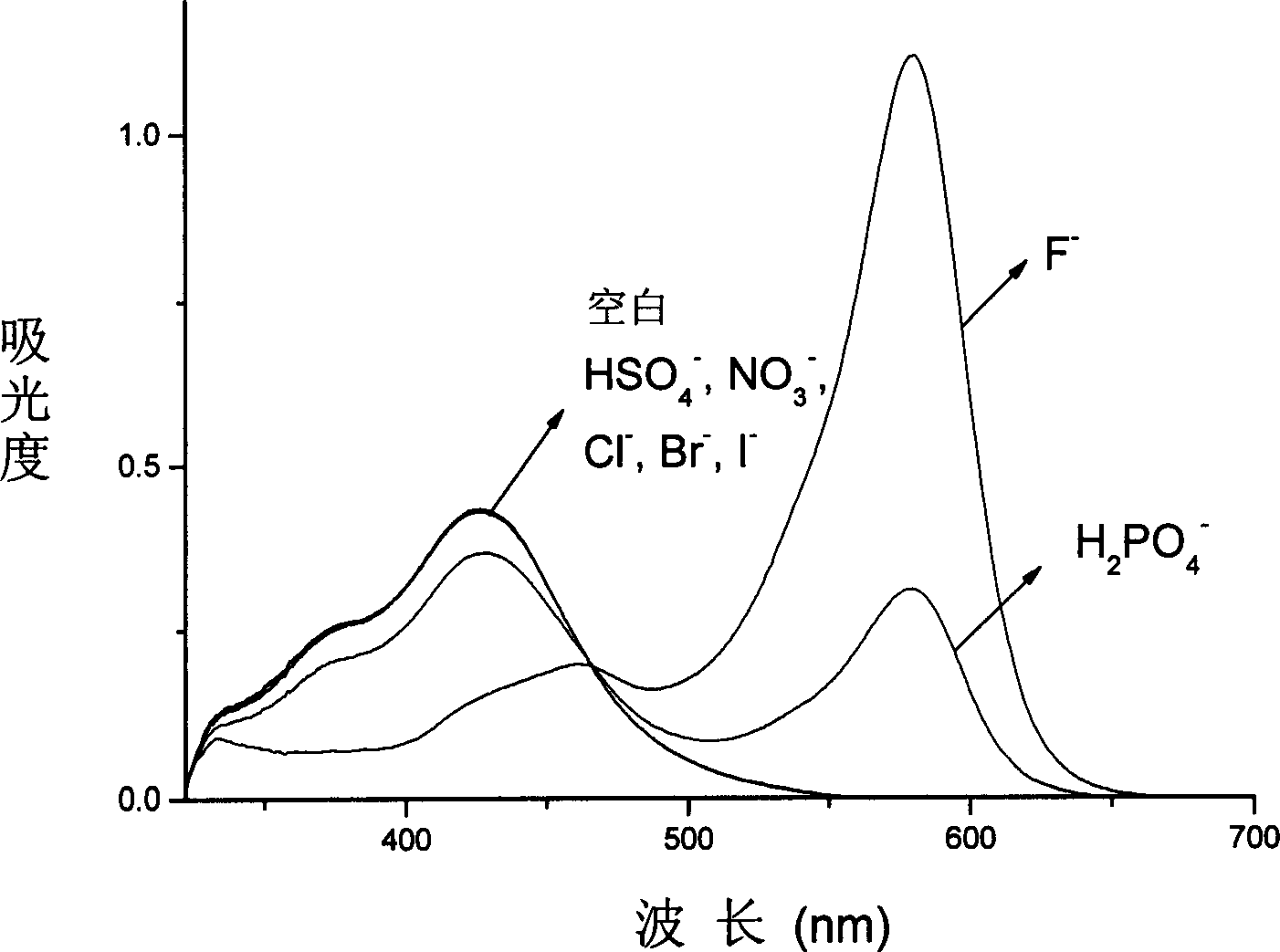
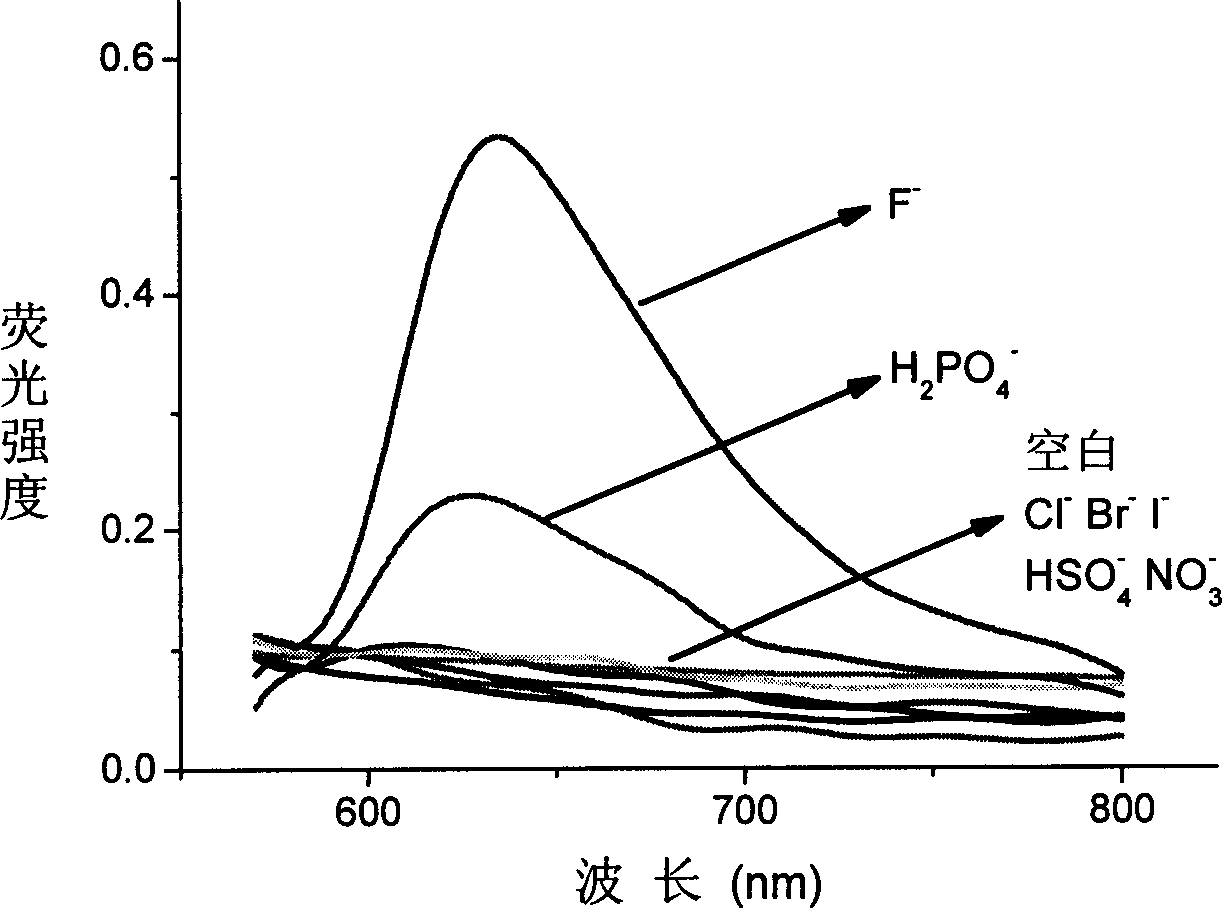
[0032] The acetonitrile solution (5 × 10 -5 M), respectively adding 1 equivalent of F - , 10 equivalents of H 2 PO 4 - , 20 equivalents of Cl - , Br - , I - , HSO 4 - , NO 3 - Tetrabutylamine salt solution, a color change is obtained (attached figure 1 ) and measure the absorption spectrum (attached figure 2 ) and fluorescence spectra (with image 3 ): obvious color change to fluoride ion, 10 times the amount of H 2 PO 4 - There is a certain change, for 20 times the amount of Cl - , Br - , I - , HSO 4 - , NO 3 - No meaningful color change was seen.
Embodiment 3
[0033] Embodiment 3 (color reaction)
[0034] Prepare the acetonitrile solution of the coordination compound of Example 1 and p-toluenesulfonic acid (1: 1), prepare the tetrabutylamine fluorine solution, mix the two solutions, and make the concentration of the coordination compound solution be 1×10 -5 M, the change of fluorine concentration, the measured visible ultraviolet spectrum ( Figure 4 ) and fluorescence spectra ( Figure 5 ). Measure the change of absorbance value at 580nm ( Figure 6 ). The binding constant between the coordination compound of Example 1 and the fluoride ion was calculated by nonlinear least square method as log K=6.71±0.04.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com