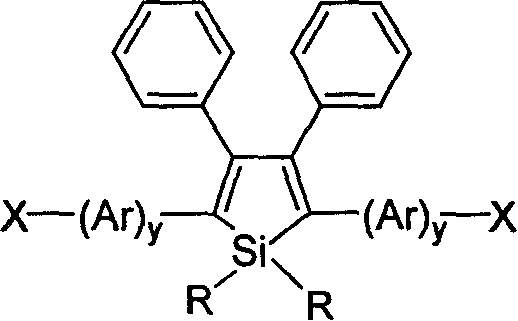
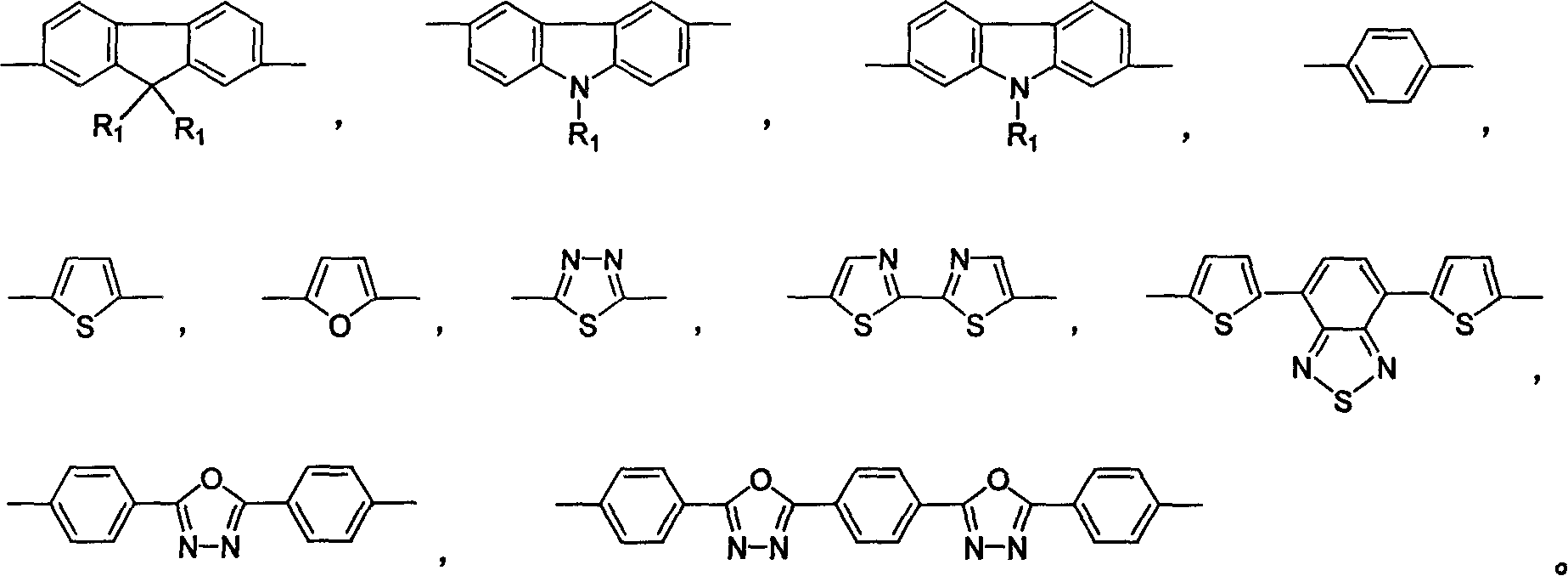
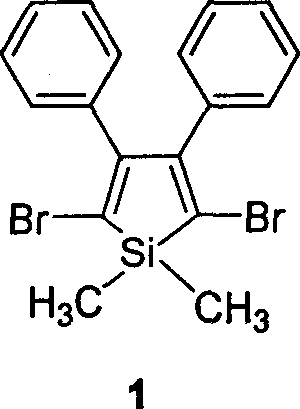
Halogen-sustituted silicon heterocycle pentadiene and production thereof
A kind of technology of silacyclopentadiene and heterocyclopentadiene, applied in new compound field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 2, the preparation of 7-dibromofluorene, reaction formula is as follows:
[0032]
[0033] 2,7-Dibromofluorene was prepared according to the method disclosed in World Patent WO 99 05184 in 1997 and the method disclosed in "Chem. Mater" (Chem. Mater) 11 (1997) 11083. Weigh fluorene (16.6 g, 0.1 mol) and iron powder (88 mg, 1.57 mmol) into a three-necked flask, add 100 ml of chloroform, cool in an ice-water bath, add bromine (35.2 g, 0.22 mol) and 35 milliliters of chloroform, the temperature in the bottle should not exceed 5°C during the dropwise addition. After the reaction was completed, it was filtered and recrystallized with chloroform to obtain white crystals (26.9 g, 83%). through 13 C NMR and GC-MASS tests showed that it was the target product 2,7-dibromofluorene.
Embodiment 2
[0035] The preparation of 2,7-dibromo-9,9-disubstituted fluorene, the reaction formula is as follows:
[0036]
[0037] The preparation of 2,7-dibromo-9,9-di-n-octylfluorene is taken as an example to illustrate. 2,7-dibromo-9,9-di-n-octylfluorene was prepared according to the method disclosed in World Patent WO 9905184 in 1997 and the method disclosed in "Chem. Mater" (Chem. Mater) 11 (1997) 11083. Get 2,7-dibromofluorene (9.72 grams, 0.03 moles) obtained in Example 1, and pour benzyltriethylammonium chloride (0.07 grams, 0.3 mmoles) into a three-necked flask, and add 90 milliliters of dimethyl sulfoxide , 45 ml of 50% aqueous sodium hydroxide solution by weight, stirred vigorously at room temperature to form a suspension, added dropwise 1-bromo-n-octane (12.5 g, 65 mmol), continued to stir for 3 hours, then extracted with ether, combined ether phase, washed with saturated aqueous sodium chloride, and dried over anhydrous magnesium sulfate. The solvent was evaporated, and...
Embodiment 3
[0040] 3, the preparation of 6-dibromocarbazole, reaction formula is as follows:
[0041]
[0042] Add carbazole (12.54 grams, 75 mmoles), 375 milliliters of refined carbon disulfide and 24 milliliters of anhydrous pyridine in a 1000 milliliter three-necked flask, stir with a mechanical stirrer while cooling with ice water, when cooled to 0 ° C, start to drop Liquid bromine (28.30 g, 177 mmol) dissolved in 75 ml of carbon disulfide was added dropwise for about 1 hour. After dropping, remove the cooling device, gradually raise to 15°C, keep stirring at 15°C for 2.5 hours, and the reaction ends. The reaction solution was poured into 400 ml of dilute hydrochloric acid, a pale yellow precipitate formed, filtered, washed with dilute sodium hydroxide solution 3 times, then washed with distilled water until neutral, and dried. Recrystallized with absolute ethanol and dried to obtain white needle-like crystals with a yield of 83%. through 1 HNMR and GC-MASS tests showed that it ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com