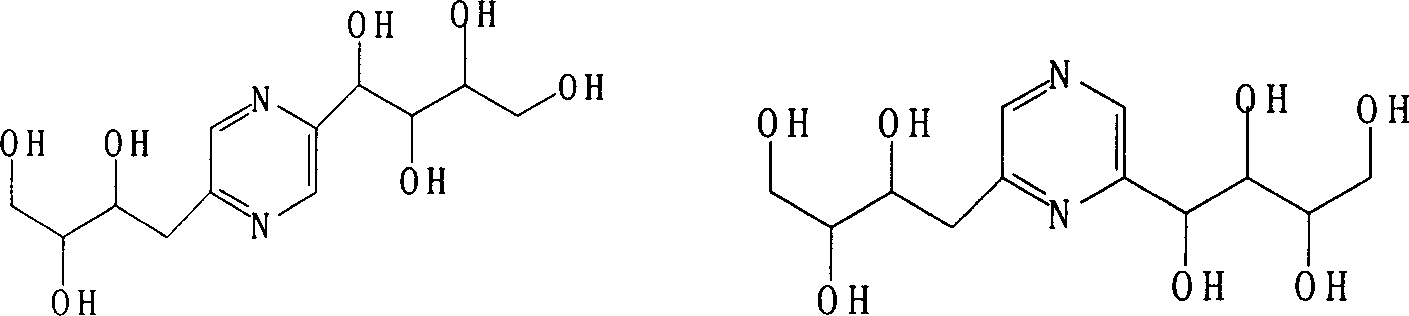
Process for preparing deoxy fructosazine
A technology of deoxyfructozine and glucosamine, which is applied in the field of preparation of deoxyfructozine, can solve the problems of unreachable industrial production, low DOF conversion rate, and difficult separation, so as to save power consumption and obtain simple and easy reaction raw materials , The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
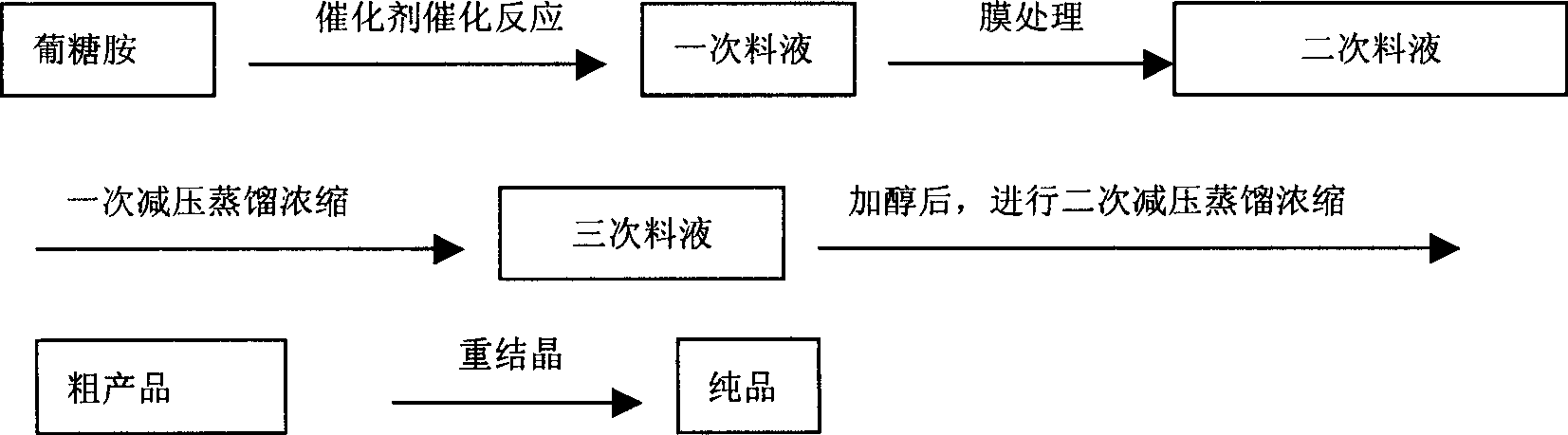
[0037] Preparation of deoxyfructosine by combining glucosamine hydrochloride boric acid-catalyzed self-condensation reaction with membrane separation technology
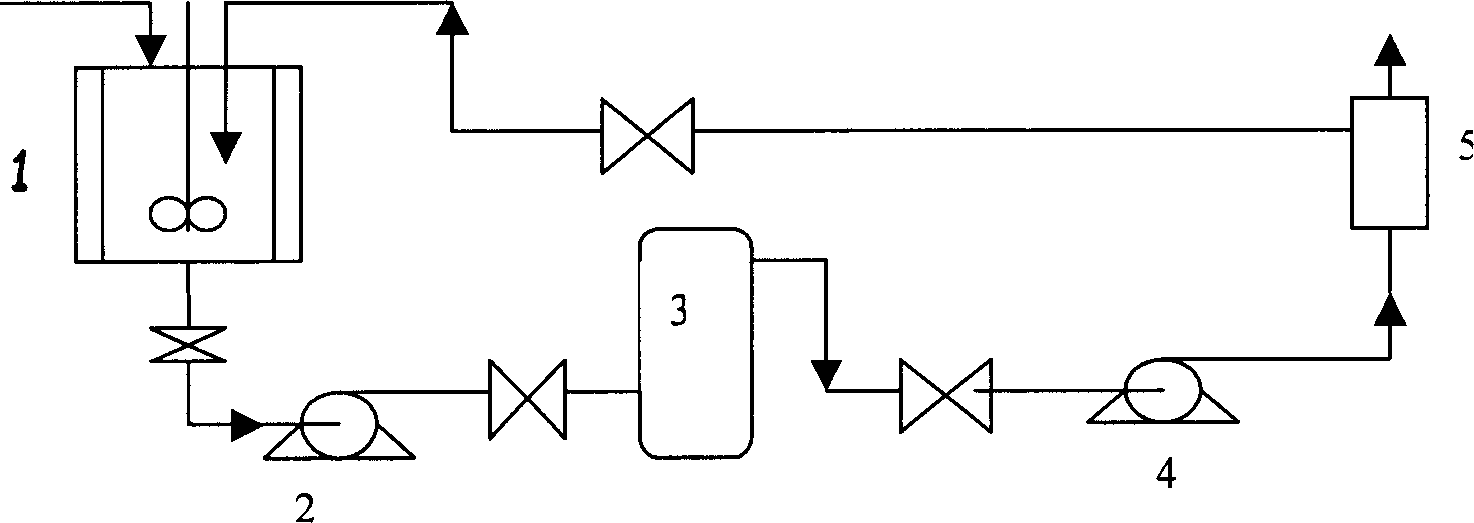
[0038]In a 30L reactor, add 12.5mol of boric acid, 500g of NaOH and 25L of water in a molar ratio of 1:1. After stirring until dissolved, add 5 mol of glucosamine hydrochloride (or glucosamine sulfate) gradually. After stirring at 15-50°C for 4 hours, 10% HCl was gradually added to adjust the pH to 2-3. The obtained mixture feed liquid is filtered through a filter bag, and then pressed into a nanofiltration membrane with a molecular weight cut-off of 80 through a high-pressure pump to remove impurities such as inorganic salts in the mixture to obtain a membrane retentate containing DOF, which does not pass through the membrane. The membrane intercepted feed liquid of the module is sent back to the raw material storage tank through the pipeline, and then sent to the membrane module for separation again. As the separa...
Embodiment 2
[0041] Preparation of deoxyfructosazine by combining glucosamine hydrochloride phenylboronic acid-catalyzed self-condensation reaction with membrane separation technology
[0042] In a 30L reactor, add 12.5mol of phenylboronic acid, 500g of NaOH, and 25L of water in a molar ratio of 1:1. After stirring until dissolved, add 5 mol of glucosamine hydrochloride gradually. After stirring at 15-50°C for 4 hours, 10% HCl was gradually added to adjust the pH to 2-3. Phenylboronic acid is precipitated from the solution, filtered, and the white solid obtained is recovered phenylboronic acid. The obtained mixed feed liquid is filtered through a filter bag, and then pressed into a nanofiltration membrane with a molecular weight cut-off of 150 through a high-pressure pump to remove impurities such as inorganic salts in the mixture to obtain a membrane retentate containing DOF, which does not pass through the membrane. The membrane intercepted feed liquid of the module is sent back to the...
Embodiment 3
[0044] Preparation of deoxyfructosazine by combining glucosamine sodium perborate catalyzed self-condensation reaction with membrane separation technology
[0045] In a 30L reaction kettle, add 12.5mol of sodium perborate and 25L of water, and add an appropriate amount of hydrochloric acid while stirring until the solution is completely clear. After dissolving, add 5 mol of glucosamine gradually. After stirring at 15-50°C for 4 hours, 10% HCl was gradually added to adjust the pH to 2-3. The obtained mixed feed liquid is filtered through a filter bag, and then pressed into a nanofiltration membrane with a molecular weight cut-off of 150 through a high-pressure pump to remove impurities such as inorganic salts in the mixture to obtain a membrane retentate containing DOF, which does not pass through the membrane. The membrane intercepted feed liquid of the module is sent back to the raw material storage tank through the pipeline, and then sent to the membrane module for separati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com