Cosmetic compositions containing fullerene clusters
A cosmetic composition and composition technology, applied in the direction of cosmetics, cosmetics, medical preparations containing active ingredients, etc., can solve the problem of reducing the number of double bonds, reducing the specific reactivity of fullerenes, and no one has successfully prepared fullerenes, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
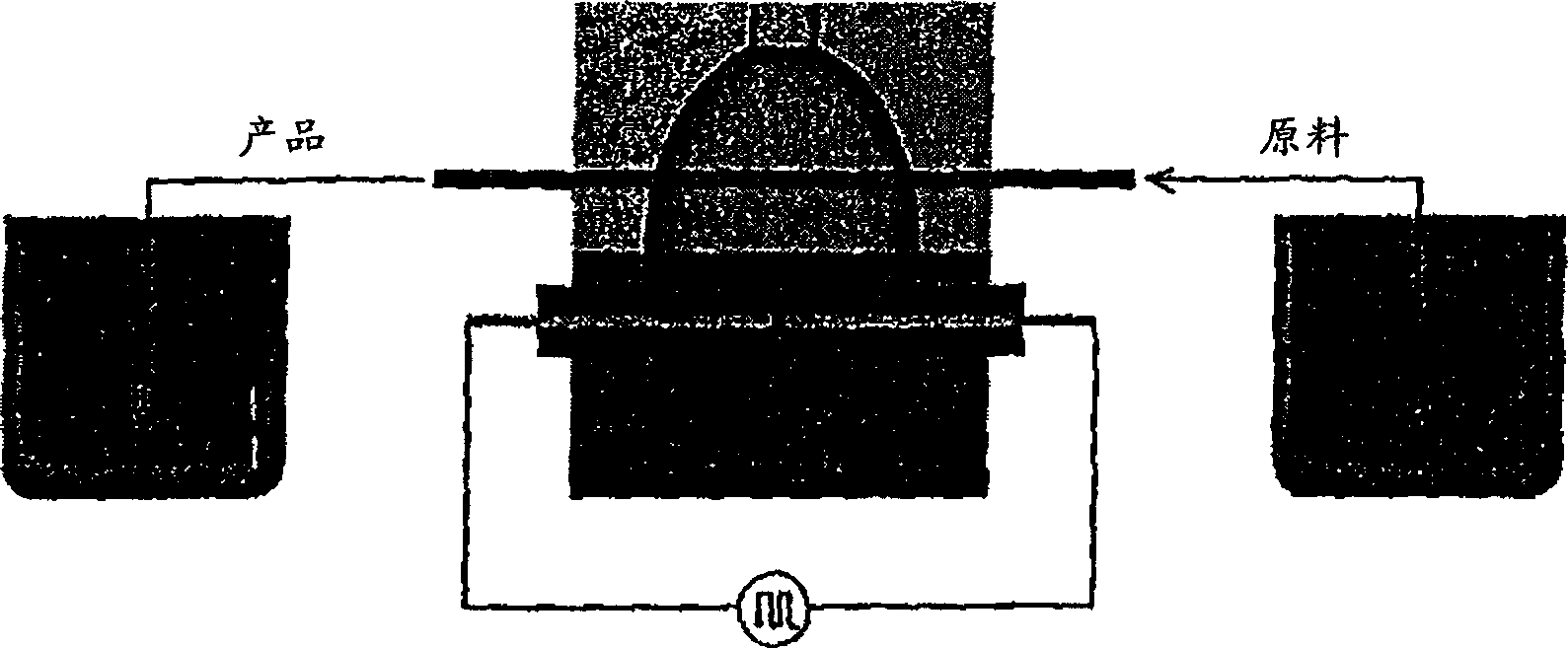
[0200] Will C 60 Vacuum sublimation of the crystalline solvate of , followed by grinding in an agate mortar in an argon-dried box to crystallites averaging about 10 μm yielded a solvent-free fullerene C with a purity of 99.9% by weight 60 . 400 mg of this powder was added to a thin-walled polyethylene test tube, followed by deionized (DI) water to 3 ml, and the open end heat sealed. Pass the sample thus prepared through an electrohydraulic device ( figure 2 ) through the small hole in the upper cover, which is inserted into a cavity filled with deionized water, which is circulated through the cooler. Fix the sample with a special fixture so that the fullerene powder is located at the upper focus of the ellipsoid. A series of discharges at a pulse frequency of 2 Hz were performed at the lower focus of the ellipsoid for a total of 2 hours, with a 5 μF capacitor charged to 6,000 V between pulses with a high-voltage power supply. The device produced about 75J of energy per pu...
Embodiment 2
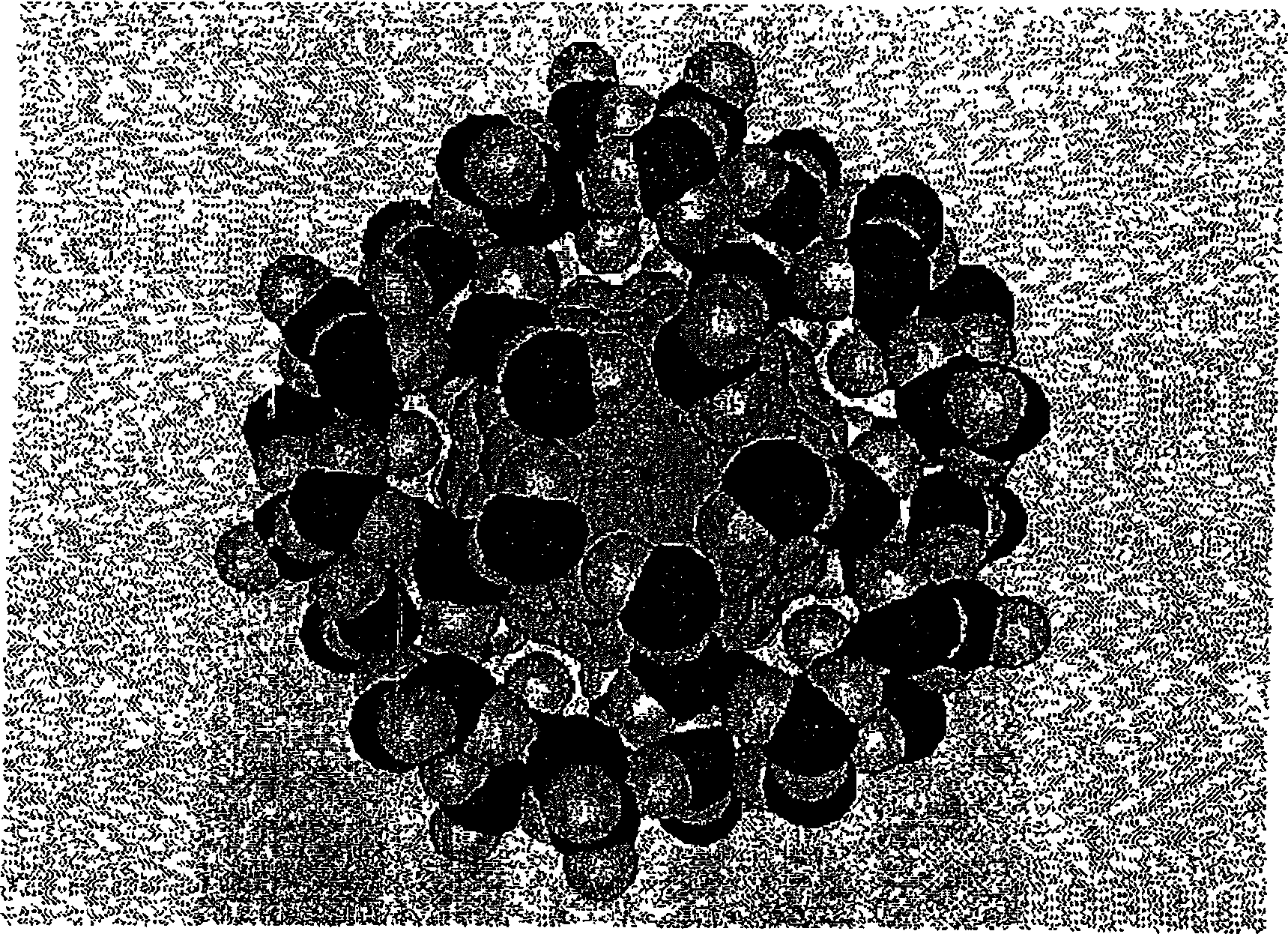
[0202] For 400 mg of fullerene C with a purity of 99.9% by weight 60 The suspension in 3 ml of clarified olive oil was subjected to the same EH treatment as in Example 1, except that the total treatment time was 4 hours. The process must be interrupted several times to adjust the gap between the electrodes, which increases due to spark erosion of the electrodes. The prepared product was diluted with ethanol at 1:10,000, and then a small drop of ethanol solution on a polished aluminum carrier was dried to obtain a sample. High-resolution SEM examination showed that there were fullerene clusters with an average diameter of about 20 nm in the sample. The prepared product is stored in the refrigerator and is easily dissolved in a large amount of olive oil or other natural oils that can be used cosmetically under gentle sonication.
Embodiment 3
[0204] 100 mg of finely ground 99.9% pure fullerene C by magnetic stirrer 60 The slurry in 200 ml of reagent grade ethanol was maintained, suspended, in a two-necked glass flask, which was further circulated with a peristaltic pump at a rate of 20 ml / min in a closed loop comprising PTFE thin tubes passing through figure 2 The upper focus of the middle ellipsoid cavity. The EH treatment conditions are the same as those in Example 1, except that the treatment time is 4 hours. After the product was filtered through a 0.22 μm PTFE filter, a reddish milky white solution was obtained with a fullerene concentration of about 0.25 mg / ml. SEM studies confirm that there are a large number of fullerene clusters with a diameter of 7-30nm and larger clusters with a diameter of 50-150nm, which account for about 1 / 3 of the mass of the entire sample. Aliquots of the product were stored in the refrigerator without signs of precipitation for at least 5 months. Additional prolonged sonication...
PUM
| Property | Measurement | Unit |
|---|---|---|
| capacitance | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com