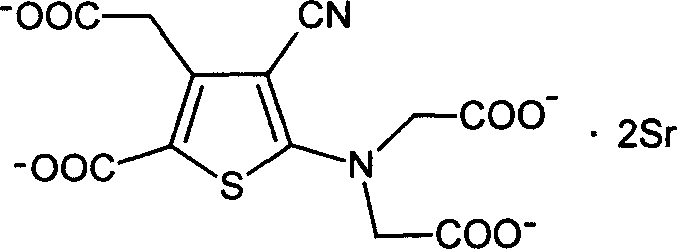
Strontium ranelate chewing tablet and its preparation process
A technology of strontium ranelate and chewable tablets, which is applied in the directions of pill delivery, bone disease, and drug combination, etc., can solve problems such as inconvenience of patients, and achieve the effect of reducing packaging volume, easy to take, and controllable product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] prescription:
[0028] Strontium ranelate 1000g
[0029] Mannitol 500g
[0030] Aspartame 30g
[0031] 10% starch slurry appropriate amount
[0032] Sweet Orange Flavor 50g
[0034] Made into 1000 pieces
[0035] Weigh the raw and auxiliary materials of the above prescription amount and pass through 100 mesh sieve respectively, prepare starch into 10% starch slurry for later use; mix strontium ranelate, mannitol, and aspartame evenly, add appropriate amount of 10% starch slurry to make soft Material, passed through a 20-mesh sieve to granulate, dried at 60-80°C, and then passed through a 20-mesh sieve for granulation. The dry granules are mixed with sweet orange essence and magnesium stearate, mixed evenly, and then compressed into capsule-shaped tablets.
Embodiment 2
[0037] prescription:
[0038] Strontium ranelate 1000g
[0039] Lactose 500g
[0040] Glycyrrhizin 30g
[0041] Sodium cyclamate 15
[0042] 5% polyvinylpyrrolidone 50% ethanol solution appropriate amount
[0043] Apple essence 50g
[0045] Made into 1000 pieces
[0046] Weigh the raw and auxiliary materials of the above-mentioned prescription quantity and pass through 100 mesh sieve respectively, and prepare a 5% solution of polyvinylpyrrolidone with 50% ethanol for later use; mix strontium ranelate, lactose, glycyrrhizin, and sodium cyclamate evenly, and add An appropriate amount of 5% polyvinylpyrrolidone 50% ethanol solution is made into a soft material, passed through a 20-mesh sieve to granulate, dried at 60-80°C, and then passed through a 20-mesh sieve for granulation. The dry granules are mixed with apple essence and talcum powder, mixed evenly, and then pressed into oval tablets.
Embodiment 3
[0048] prescription:
[0049] Strontium ranelate 1000g
[0050] Compressible starch 170g
[0051] Microcrystalline Cellulose 100g
[0052] Xylitol 200g
[0053] Stevia 30g
[0054] Strawberry essence 50g
[0057] Made into 1000 pieces
[0058] Weigh the raw and auxiliary materials of the above prescription amount, pass through a 100-mesh sieve, mix strontium ranelate, compressible starch, microcrystalline cellulose, xylitol, stevioside, and strawberry flavor evenly, and then mix them with magnesium stearate Mix evenly with Monascus pigment, and then directly press into heart-shaped tablets weighing about 1.6g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com