Metal microparticle, composition containing the same and process for producing metal microparticle
A technology of metal particles and manufacturing methods, applied in the field of nano-sized rod-shaped gold particles, metal particle compositions, and metal particle manufacturing, which can solve problems such as slow reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Method for producing gold particles
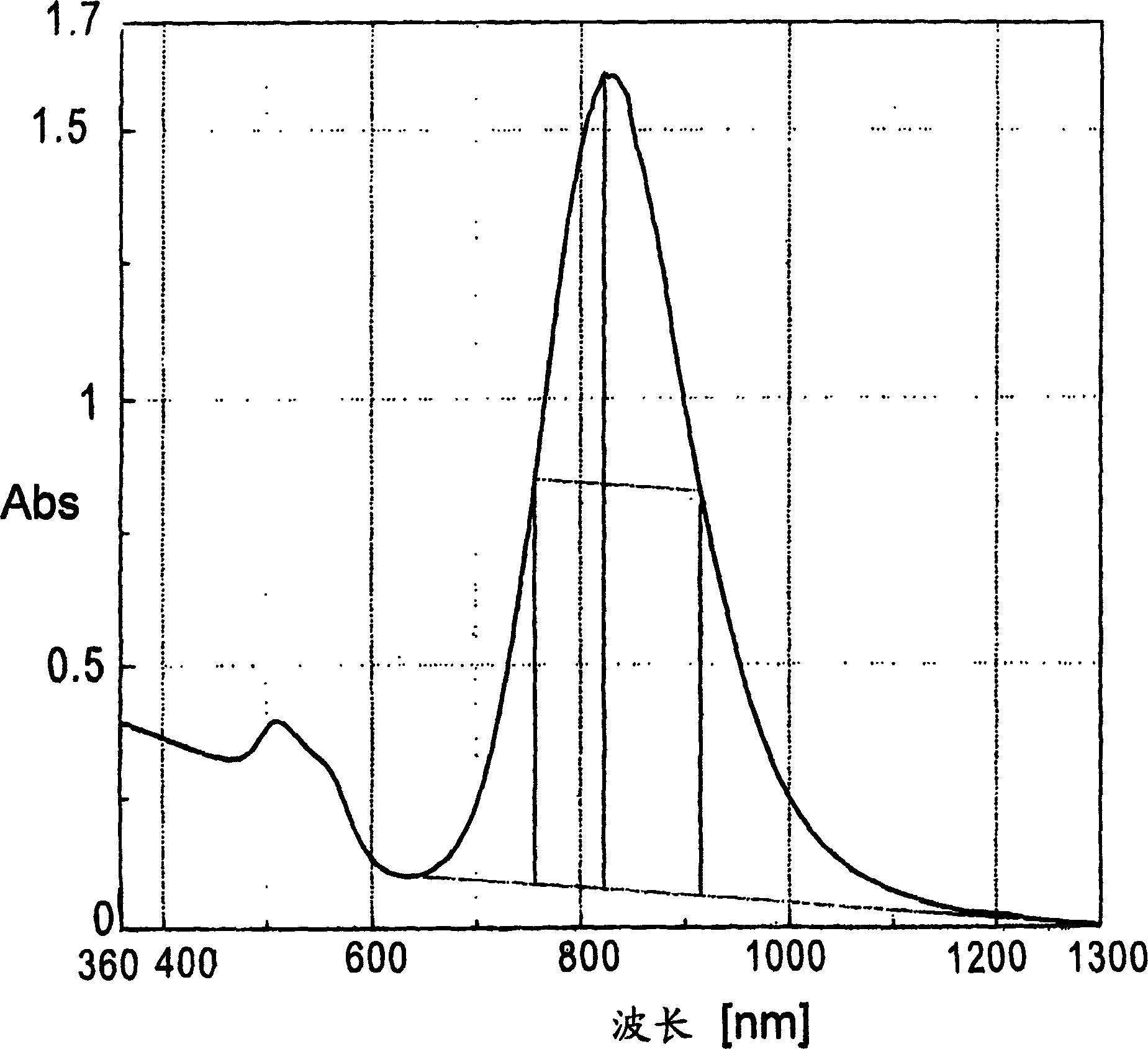
[0111] To 50 ml of 0.50 mol / L CTAB aqueous solution, 5 ml of 24 mmol / L aqueous chloroauric acid solution, 1 ml of acetone, 1 ml of cyclohexane, 1 ml of cyclohexanone, and 5 ml of 10 mmol / L aqueous silver nitrate solution were added to prepare a reaction solution. To this reaction solution, 5 ml of a 40 mmol / L ascorbic acid (AS) aqueous solution was added to perform chemical reduction. The reaction solution changed from orange to a clear solution immediately after the AS aqueous solution was added. The transparent solution was put into a beaker with a capacity of 100 ml, and the synthesis solution was directly irradiated with ultraviolet rays from a UV irradiator (high pressure mercury lamp) from the upper part of the beaker for 5 minutes. After being irradiated with light, it was left as it was, and after 1 hour, it was transferred to a storage container, and the solution was diluted 10 times with water (volume ratio, gold particle...
Embodiment 2
[0114] Gold particles surface treated with dispersant
[0115] 0.1 g of a dispersant (manufactured by Avecia: Solsperse 24000SC) was dissolved in 10 g of toluene, and the gold nanorods synthesized in Example 1 (average length of short axis: 10 nm, average length of long axis: 42 nm, Aspect ratio 4.2) 50 g of the aqueous dispersion was stirred for 10 minutes with a mixer (rotational speed 300 rpm). To this solution, 30 g of ethanol was added, and the solution was left to stand for 24 hours. By adding ethanol, the solubility of CTAB becomes high, the CTAB adsorbed on the surface of the gold nanorods is desorbed, and the nitrogen site of the dispersant is adsorbed on the gold nanorods and replaced with CTAB, and the surface treatment is performed accordingly.
[0116] The standing mixture was separated into a colorless and transparent aqueous phase and a bright red toluene phase. Then, only the organic solvent layer was extracted, and the remaining toluene was removed using an ...
Embodiment 3
[0119] Gold particle composition and film
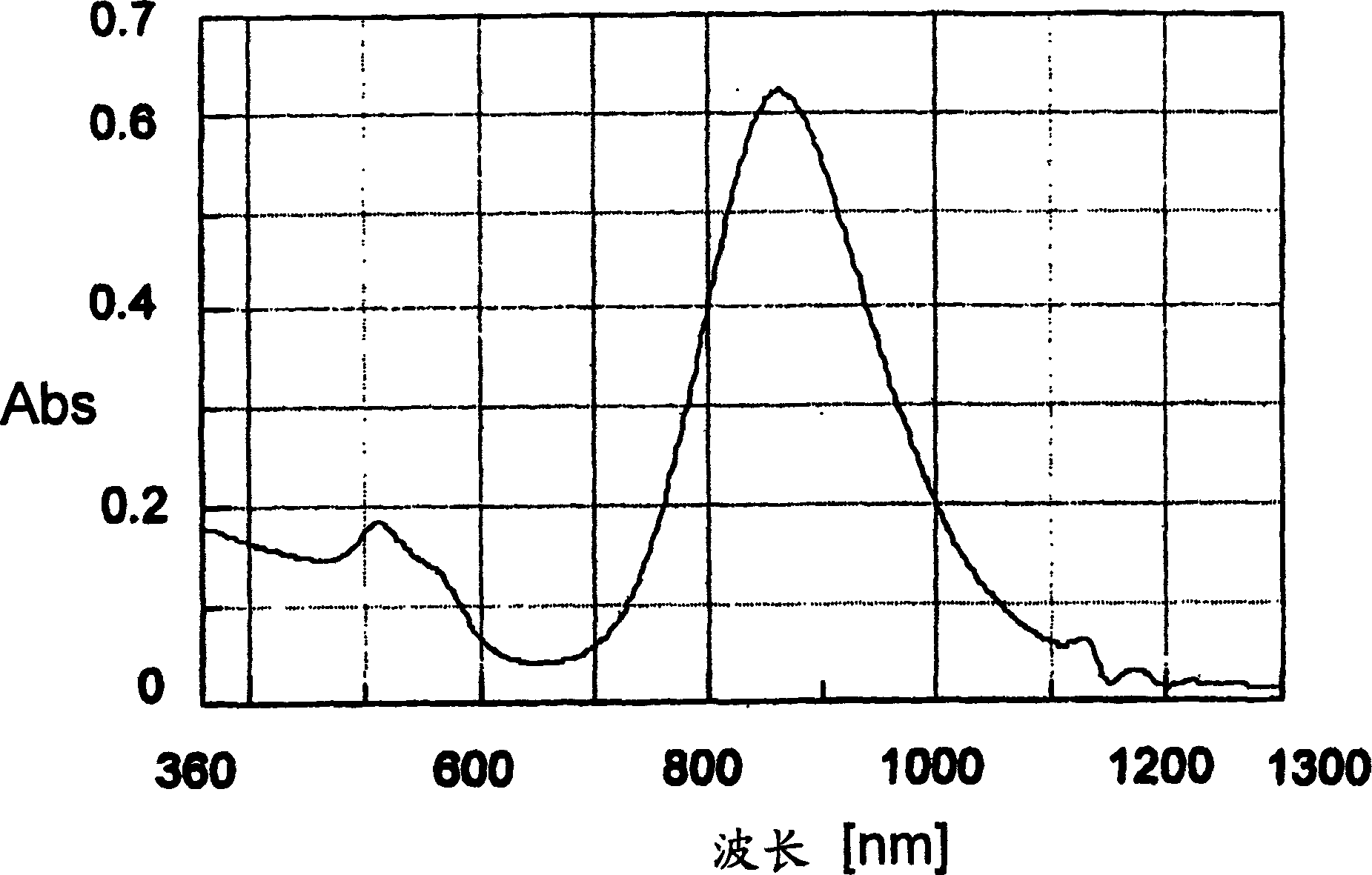
[0120] 5 g of the gold nanorod concentrate of Example 2 was mixed with 20 g of a mixture of a radically polymerizable urethane-based oligomer and a radical polymerization initiator to prepare a paint. The paint was kept at room temperature for 3 months or more in a light-shielded state without discoloration or precipitation, and was stable.
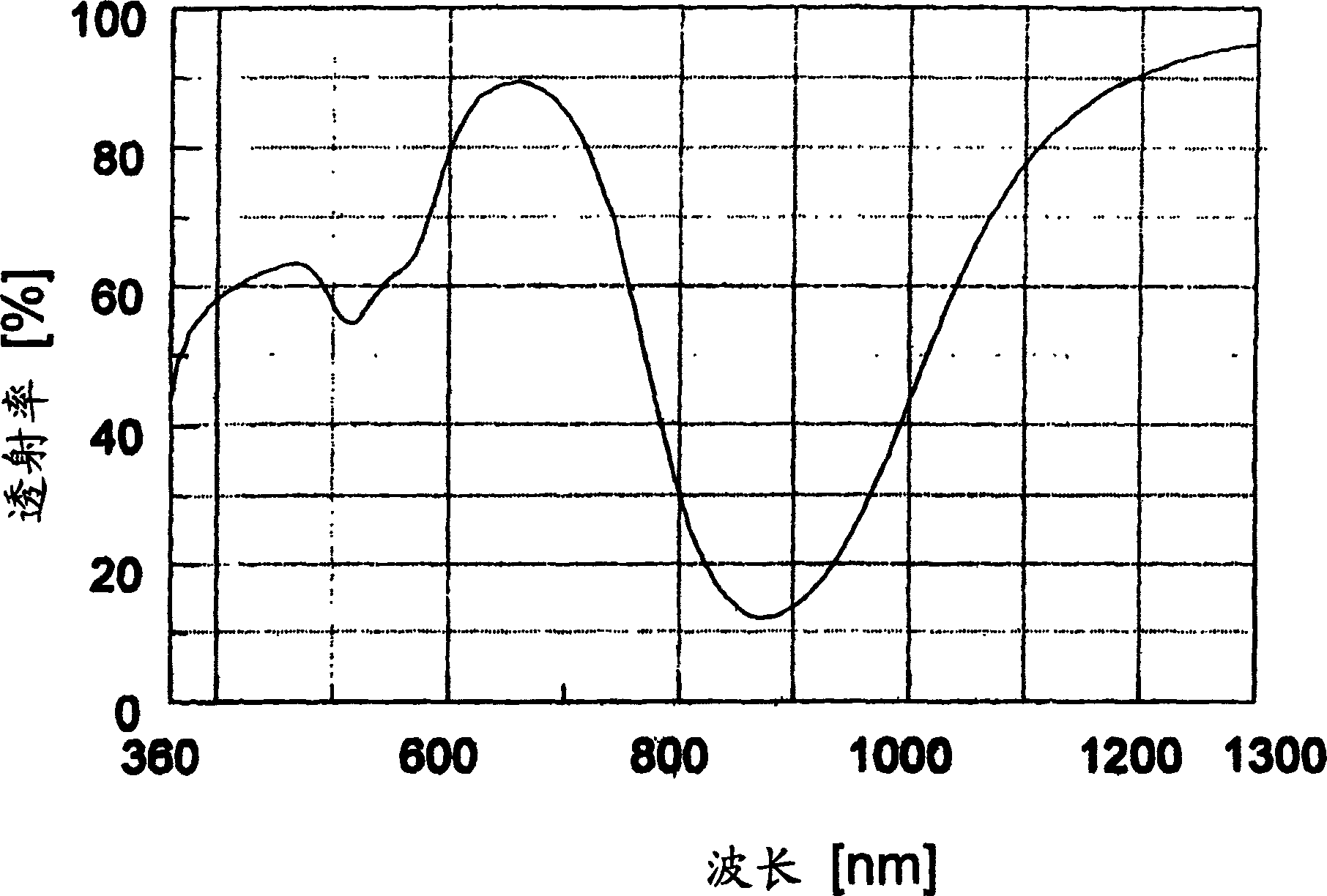
[0121] This coating material was applied on a glass plate (the content of gold fine particles was 1 wt %, and the dry film thickness was 10 μm), and the transmission spectrum was measured. image 3 shows this result. As shown in the figure, with figure 2 The peak position of the peak position of the absorption maximum wavelength is the lowest in the vicinity of the wavelength (870 nm), which confirms that the specific wavelength is absorbed by the gold nanorods.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Absorption coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com