Dopamine transfer protein peptide inhibitor and its use
A transporter and inhibitor technology, applied in the field of pharmaceuticals, can solve problems such as lack of dopamine transporter
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Example 1 Design of β-transition peptide
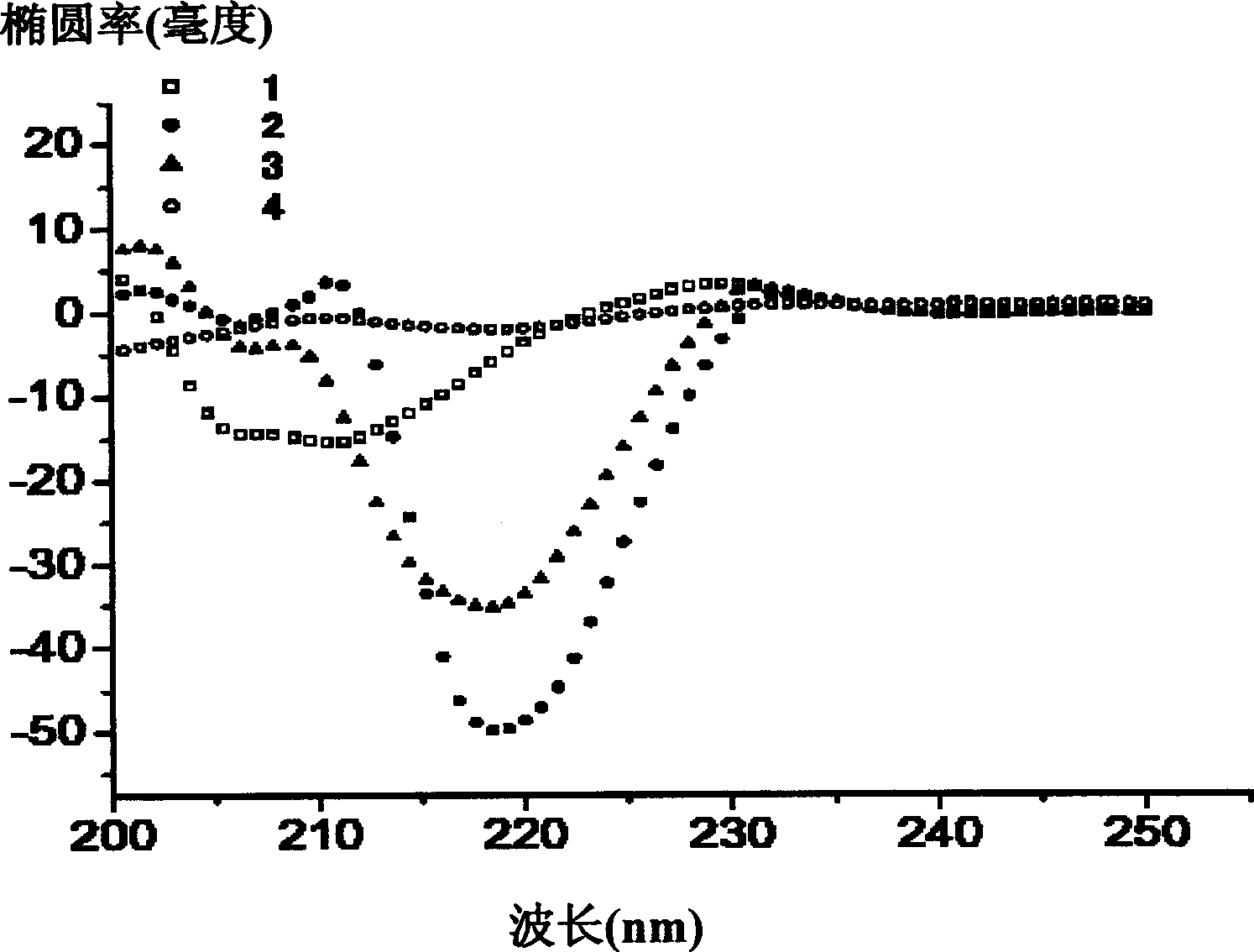
[0088] A variety of short peptides shown in Table 1 (peptides 1-9 have the sequence shown in SEQ ID NO:1-9 respectively) were designed and synthesized by conventional methods. Then use far ultraviolet circular dichroism to determine the secondary structure of the peptidomimetic. Methods as below:
[0089] The peptidomimetic was dissolved in 20 mmol potassium phosphate, 40 mmol sodium chloride, pH 7 solution containing 10% trifluoroethanol, and the peptide concentration was 0.4 mg / ml. The model of the circular dichrograph is Jasco J-715, and the test is carried out at 20℃, using standard parameters: wavelength is 190-250nm; sensitivity is 20mdeg; data collection interval is 0.1nm; scanning speed is 10nm / s; cumulative value 1; The response time is 0.25 seconds; the bandwidth is 1nm; the optical path is 0.1cm. The β-turn content in the peptidomimetic is positively correlated with the ellipticity at 218nm.
[0090] The result is figure...
Embodiment 2
[0096] Example 2 Structure-function relationship of peptidomimetic
[0097] In Table 1, peptides 1-4 clearly show that the β-turn is directly related to the activity of the peptidomimetic. Peptide 5 is designed on the basis of peptide 3 and has a similar secondary structure, but the activity is increased by more than 50 times. This is related to the improvement of the local structure which increases the binding force of the peptidomimetic and dopamine transporter, such as the removal of the basic L-lysine residue and the benzylation of the C-terminal L-threonine hydroxyl group. These changes Enhance the hydrophobicity of the molecule. The C-terminal L-glycine in peptide 5 is not necessary for the active structure, but is designed to facilitate solid phase synthesis. Peptides 7-9 show that the O-benzyl L-threonine in peptide 5 can be replaced by L-phenylalanine, L-tyrosine can be replaced by L-phenylalanine, and L-threonine can be Replaced by L-serine. These substitutions basically...
Embodiment 3
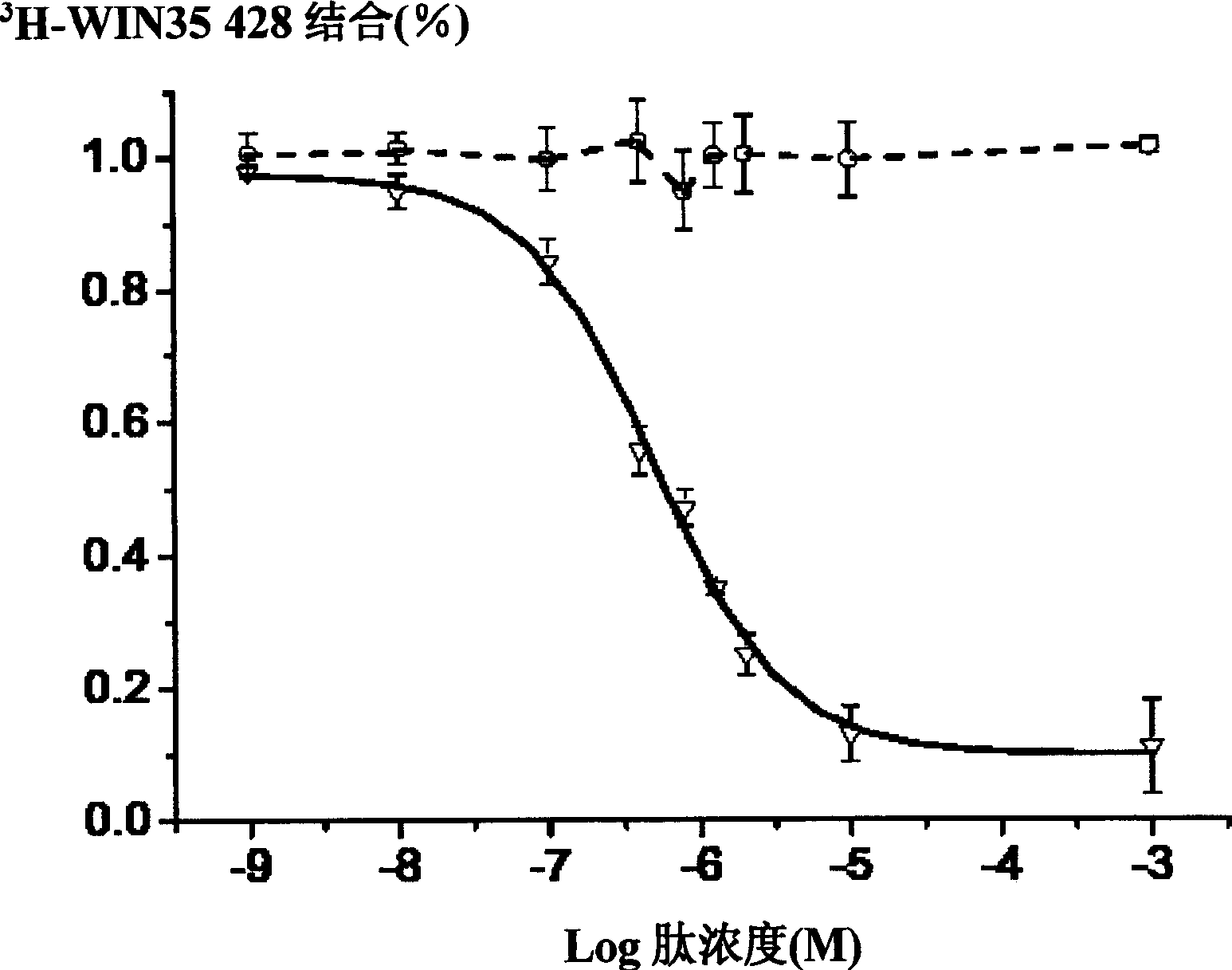
[0106] Example 3 Inhibition kinetics of peptidomimetic on dopamine transporter
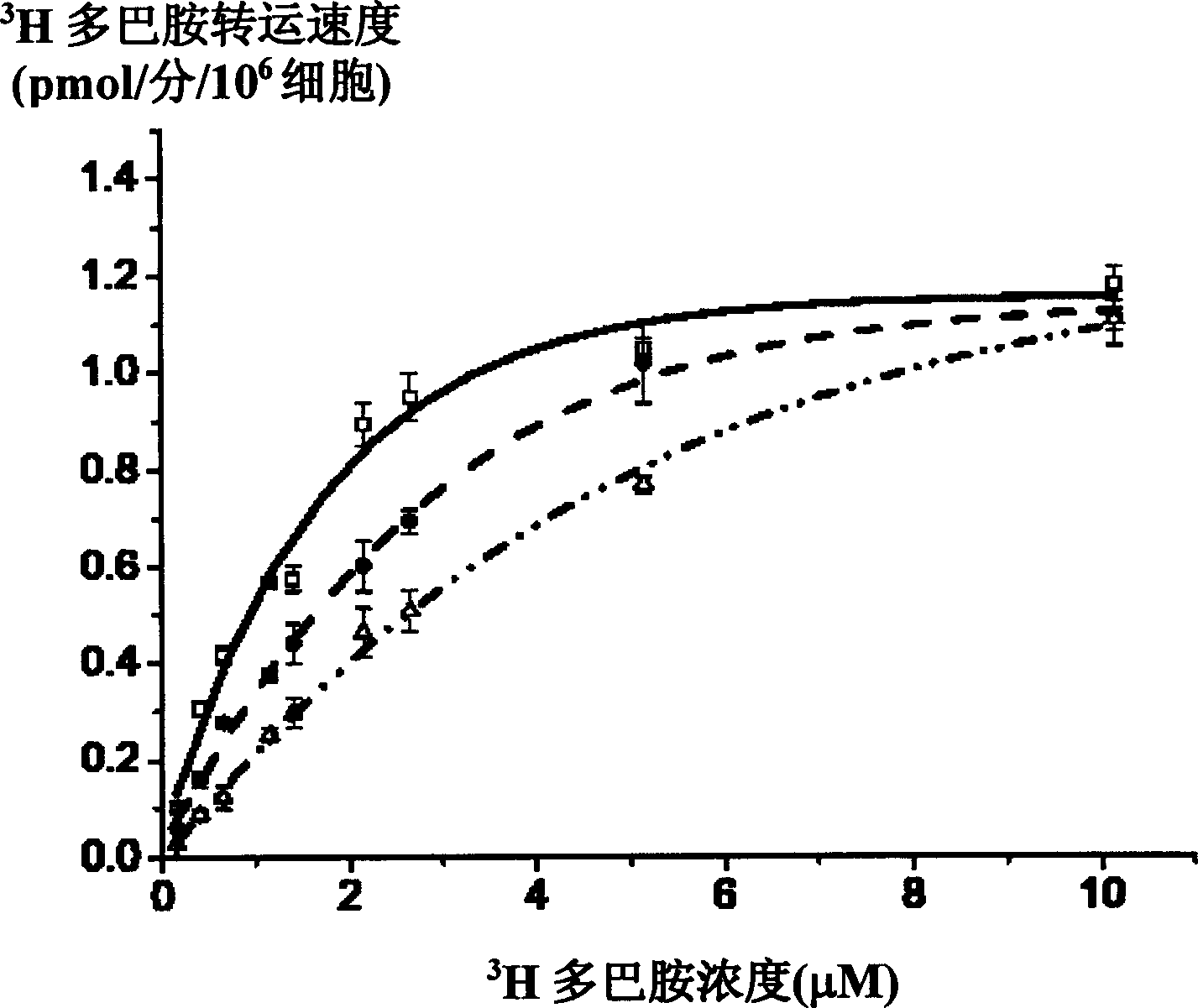
[0107] The kinetic analysis of the peptidomimetic was performed on CHO cells stably expressing rat dopamine transporter. 1μM peptide 5 can inhibit 80% of the dopamine transporter activity, and after two washings, this inhibition can be completely reversed. Therefore, peptide 5 is a complete reversible inhibitor.
[0108] Saturation analysis experiments were also used to determine the properties of peptide 5 acting on dopamine transporters ( figure 2 ). When there is no inhibitor, the Km value of dopamine transporter for dopamine transport is 1.38±0.27μM, and the maximum transport speed Vmax is 1.27±0.13pmol / min.106cells; when there is 250nM peptide 5, the Km value is 2.41±0.35μM, Vmax It is 1.29±0.11pmol / min.106cells; when there is 600nM peptide 5, the Km value is 3.59±0.20μM, and the Vmax is 1.25±0.08pmol / min.106cells. It can be seen that peptide 5 only changes the Km value without changing the Vmax...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com