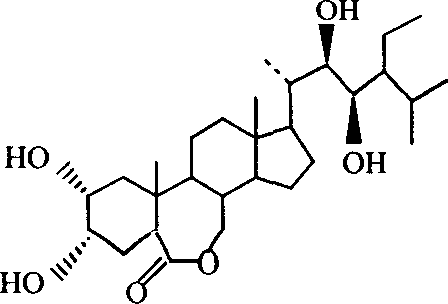
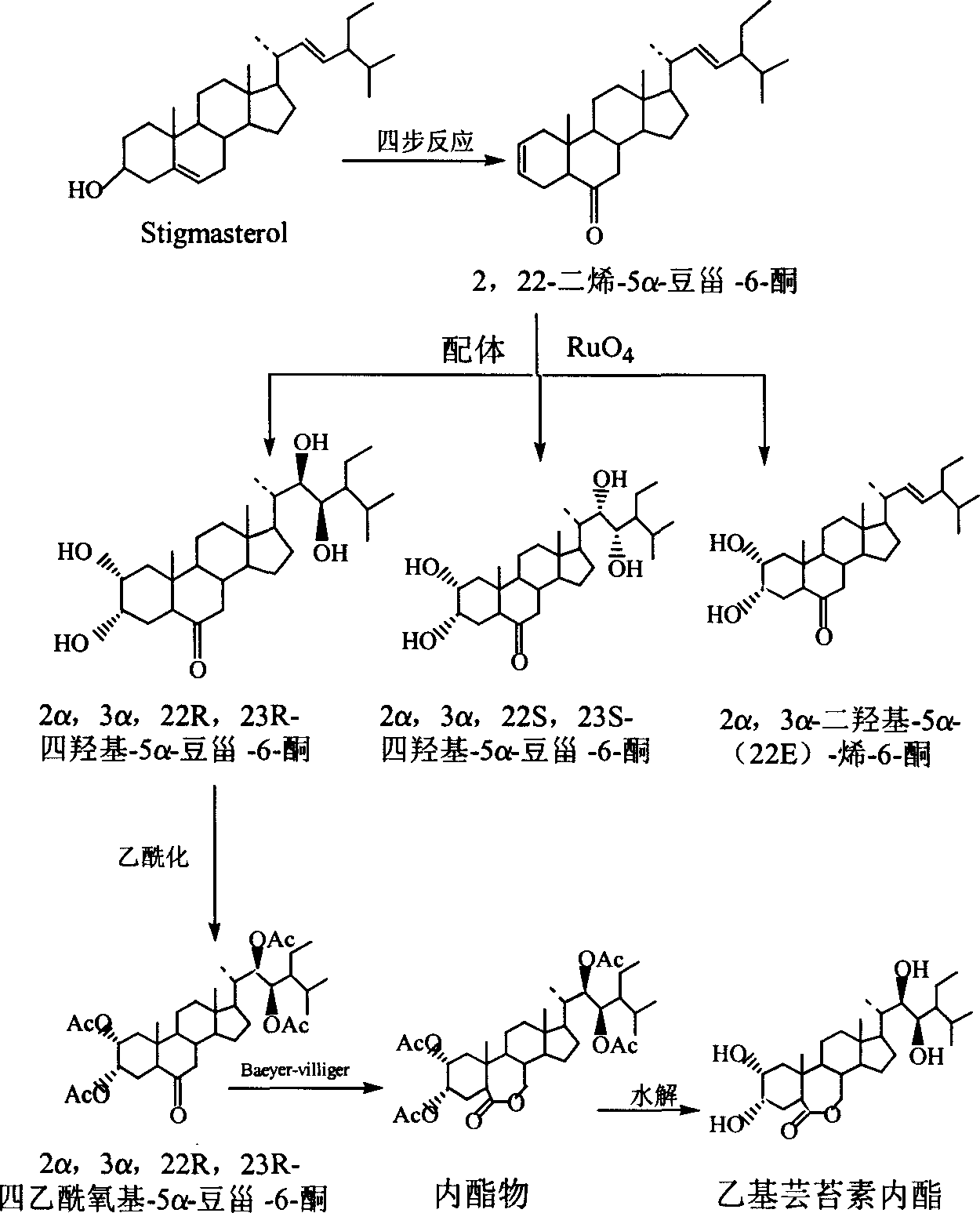
Manufacture method of ethyl brassinolide
A technology of ethyl brassinolide and ethyl, which is applied in the chemical field, can solve the problems of inability to realize industrial production, low yield of final product, and high cost, and achieve the goals of increasing yield, shortening reaction time, and improving production efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] [Example 1] Preparation of ethyl brassinolide
[0049] 1. Preparation of compound 2,22-diene-5α-stigmaster-6-one
[0050] Starting from stigmasterol, through the method of literature Chem.Pharm.Bull., 1982, 30 (11), pp4181-4185., through four-step reaction, directly obtain 2,22-diene-5α-stigmaster-6-one .
[0051] 2. Preparation of compound 2α, 3α, 22R, 23R-tetrahydroxy-5α-stigmaster-6-one
[0052] In a 3000ml three-neck flask, add 100g of 2,22-diene-5α-stigmaster-6-one, add ligand: 20g of phenanthrene-dihydroquinidine ether, stir and dissolve with 2000ml of tert-butanol, and cool to 0 ℃, slowly dropwise add 300ml of the oxidant ruthenium tetroxide prepared according to the example, keep the dropping temperature below 0℃, and then react at 0℃ for 1 hour; then at 0℃, slowly add 200g of sodium bisulfite to end the reaction. The reaction solution was dispersed in 2000ml water, extracted three times with 1000ml ethyl acetate respectively, the ethyl acetate layers were co...
Embodiment 2
[0059] [embodiment 2] the preparation of ethyl brassinolide
[0060] 1. Preparation of compound 2,22-diene-5α-stigmaster-6-one
[0061] Starting from stigmasterol, through the method of literature Chem.Pharm.Bull., 1982, 30 (11), pp4181-4185., through four-step reaction, directly obtain 2,22-diene-5α-stigmaster-6-one .
[0062] 2. Preparation of compound 2α, 3α, 22R, 23R-tetrahydroxy-5α-stigmaster-6-one
[0063] In a 3000ml three-necked flask, add 90 g of 2,22-diene-5α-stigmaster-6-one, and 2α, 3α-dihydroxy-5α-(22E)-en-6-one recovered in Example 1 10g, add ligand: 20g of phenanthrene-dihydroquinidine ether, stir and dissolve with 2000ml of tert-butanol, cool to -5°C, slowly add 300ml of oxidant ruthenium tetroxide dropwise, keep the dropping temperature less than -5°C, and then in React at -5°C for 1 hour; then at 0°C, slowly add 220 g of sodium bisulfite to end the reaction. The reaction solution was dispersed in 2000ml water, extracted three times with 1000ml ethyl aceta...
Embodiment 3
[0070] [embodiment 3] the preparation of ethyl brassinolide
[0071] 1. Preparation of compound 2,22-diene-5α-stigmaster-6-one
[0072] Starting from stigmasterol, through the method of literature Chem.Pharm.Bull., 1982, 30 (11), pp4181-4185., through four-step reaction, directly obtain 2,22-diene-5α-stigmaster-6-one .
[0073] 2. Preparation of compound 2α, 3α, 22R, 23R-tetrahydroxy-5α-stigmaster-6-one
[0074] In a 10000ml three-neck flask, add 250g of 2,22-diene-5α-stigmaster-6-one, and 50g of recovered 2α,3α-dihydroxy-5α-(22E)-en-6-one, and add Body: 60g of phenanthrene-dihydroquinidine ether, stirred and dissolved with 2000ml of tert-butanol, cooled to -15°C, slowly added dropwise 900ml of oxidant ruthenium tetroxide prepared according to Example 1, keeping the dropping temperature below -10°C, Then react at -10°C for 1 hour; then at 0°C, slowly add 220g of sodium bisulfite to end the reaction. The reaction solution was dispersed in 6000ml water, extracted three times...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com