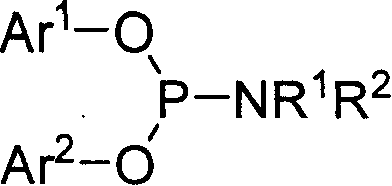
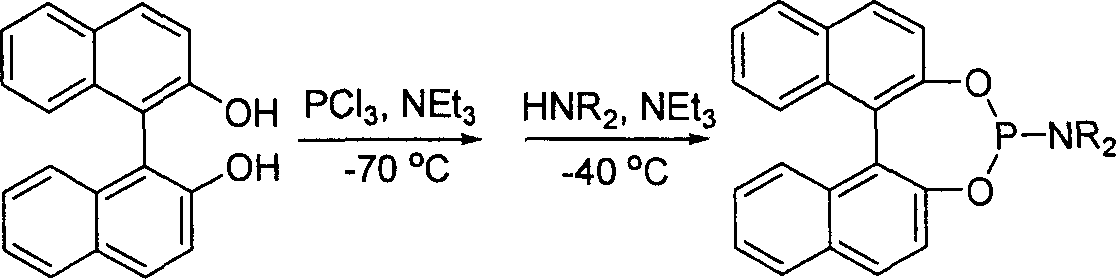
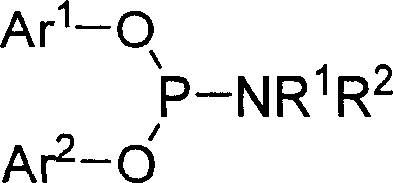
Phosphorous amide ligand, its prepn. method and application
A phosphoramidite and ligand technology, applied in the field of phosphoramidite ligands, to achieve high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Preparation of Phosphoramidite Ligands
[0024] Under the protection of argon, a mixture of 96.6 mmol of redistilled diethylamine and 100 mL of anhydrous ether was added to a 250 mL round bottom flask equipped with an electric stirrer, a thermometer and a dropping funnel. Cool it to 0°C with an ice-water bath under constant stirring, and slowly add 16.1 mmol of dry PCl dropwise 3 Add 50mL of diethyl ether solution to the reactor, and maintain the reactor temperature between 0-5°C. During the dropwise addition, white solids were continuously produced. After adding PCl dropwise 3 After solution, the reaction system was warmed to room temperature and kept stirring overnight. Remove the solid produced by the reaction by filtration, evaporate the solvent in the filtrate, add 20 mL of dry benzene to the residue, then transfer it to a 50 mL round bottom flask, add 16.1 mmol of binaphthol and 0.01 g of NH 4 Cl and a magnetic stirring bar, installed a reflux conde...
Embodiment 2
[0036] The application of the phosphoramidite ligand of the present invention in the brominated aromatic hydrocarbon Suzuki reaction, its reaction formula is:
[0037]
[0038] Specific operating conditions:
[0039] Add 1.5mmol aromatic boronic acid and 3mmol potassium phosphate to a 15mL branch tube with a screw cap. After the test tube was replaced with argon for 5 times, add 0.0005mmol palladium acetate, 0.001mmol phosphoramidite ligand 2c prepared by the method in Example 1, so that the phosphoramidite body and palladium form a complex in the reaction; 1.0mmol aryl bromide and 1mL of 1,4-dioxane solvent, the test tube was replaced with argon 5 times, sealed well, and heated and stirred at 80°C for 12 hours to complete the cross-coupling reaction. After completion of the reaction, after cooling to room temperature, add 4 mL of ethyl acetate and 10 mL of water, shake fully to separate the organic phase, extract the water phase with 3 × 10 mL of ethyl acetate for 3 times...
Embodiment 3
[0041] The application of the phosphoramidite ligand of the present invention in the aryl amination reaction of iodoarenes, its reaction formula is:
[0042]
[0043] Specific steps:
[0044] Add 0.025 mmol of cuprous bromide, 3 mmol of cesium carbonate and 0.05 mmol of phosphoramidite ligand 5d prepared by the method in Example 1 into a 15 mL branch tube with a screw cap. After the test tube was replaced with argon 5 times, 1 mmol of iodoarene, 1.5 mmol of diamine, and 1 mL of N,N-dimethylformamide (DMF) solvent were added with a syringe. After the test tube was replaced with argon 5 times, it was sealed well. Heat and stir at 90°C for 24 hours. After the reaction, cool to room temperature, add 4mL ethyl acetate and 10mL water, shake fully to separate the organic phase, extract the water phase with 3×10mL ethyl acetate for 3 times, combine the organic phases, wash with 20mL saturated brine, no water over sodium sulfate for 2 hours. Remove the solvent, use petroleum eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com