Preparation method of amorphous fluvastatin sodium
A fluvastatin sodium and amorphous technology, which is applied in the field of preparation of amorphous fluvastatin sodium, can solve the problems of low yield and achieve high yield and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
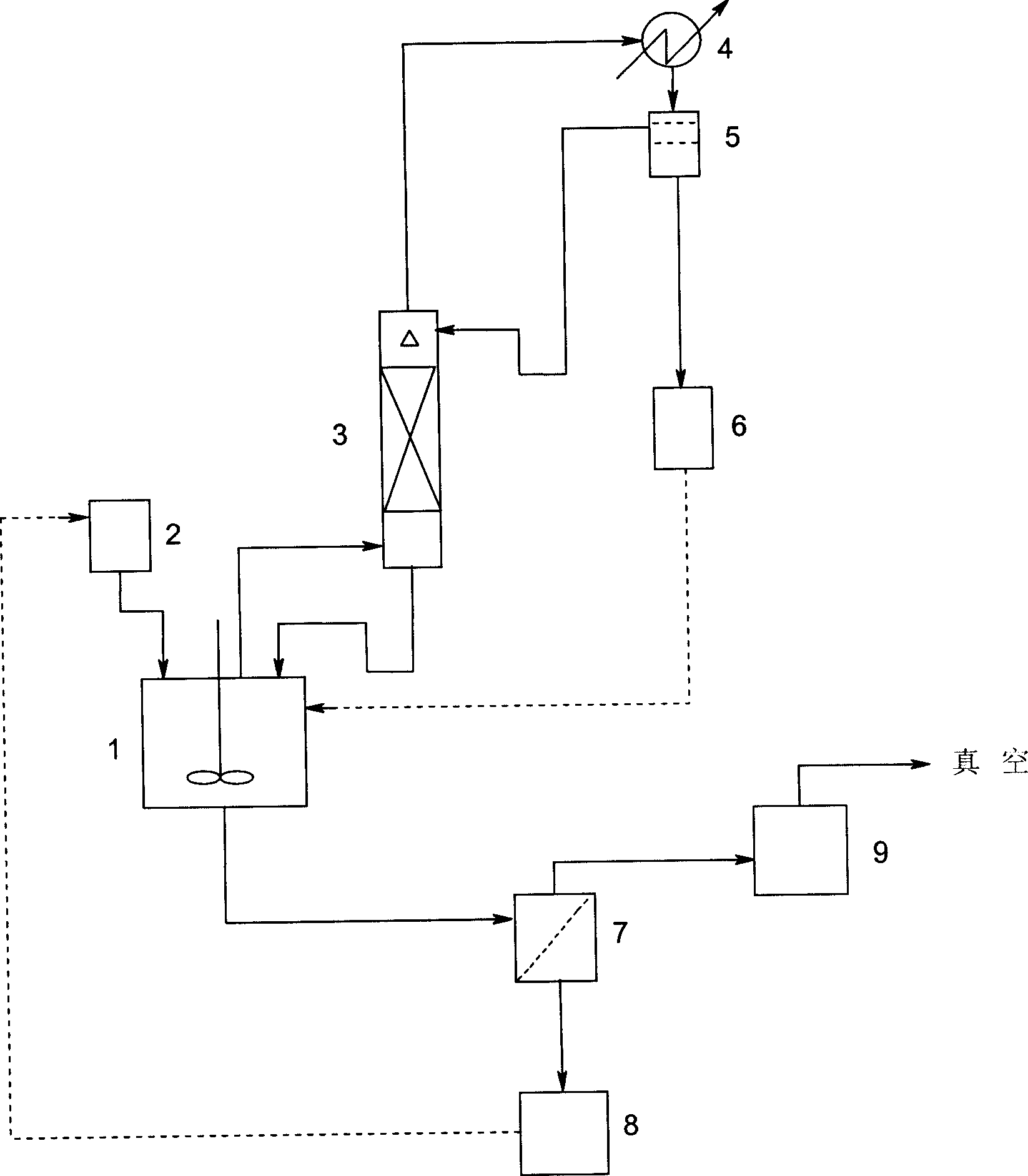
[0024] Put 20.0Kg of crystalline fluvastatin sodium into a 500L stainless steel still 1 with stirring and distillation tower 3 (diameter Ф300, equipped with 4m BX wire mesh packing), add 300L of methanol saturated with n-hexane in advance, stir and heat up Bring to a boil to completely dissolve the crystals of fluvastatin sodium. Open the alkane high tank 2 valve, add n-hexane dropwise, and control the drop rate at about 100L / hr. The azeotrope of alkane and methanol evaporated from the top of the distillation tower is condensed in the condenser 4, and the heating is adjusted so that the amount of the methanol phase in the condensate is about 100L / hr, and the stratification is carried out in the stratifier 5, and the methanol is continuously separated phase is placed in the methanol phase storage tank 6, and the upper n-hexane phase is all refluxed to the top of the distillation tower. After about 3 hours, when the temperature in the kettle rose to the boiling point of n-hexan...
Embodiment 2
[0027] In the same device as in Example 1, drop into 20.0Kg crystalline fluvastatin sodium, add about 300L of methanol phase that was separated during the last batch of distillation, stir and heat up to boiling, so that the fluvastatin sodium crystals are completely dissolved. Open the dripping valve, drip the mixed solution formed by about 250L of the filtrate in Example 1 and the added 50L n-hexane, the rate of addition is controlled at about 100L / hr, and carry out azeotropic distillation, cooling, and filtration the same as in Example 1 , drying operation, to obtain amorphous fluvastatin sodium 20.0Kg.
Embodiment 3
[0029] In the same device as in Example 1, 20.0 Kg of crystalline fluvastatin sodium was dropped into, and 300 L of methanol saturated with cyclohexane was added in advance, and when the temperature in the still was controlled to reach about 80° C., heating and dropwise addition were stopped, and the mixture was stirred under stirring. The material was cooled to 15° C., and the other operations were the same as in Example 1 to finally obtain 19.8 Kg of amorphous fluvastatin sodium.
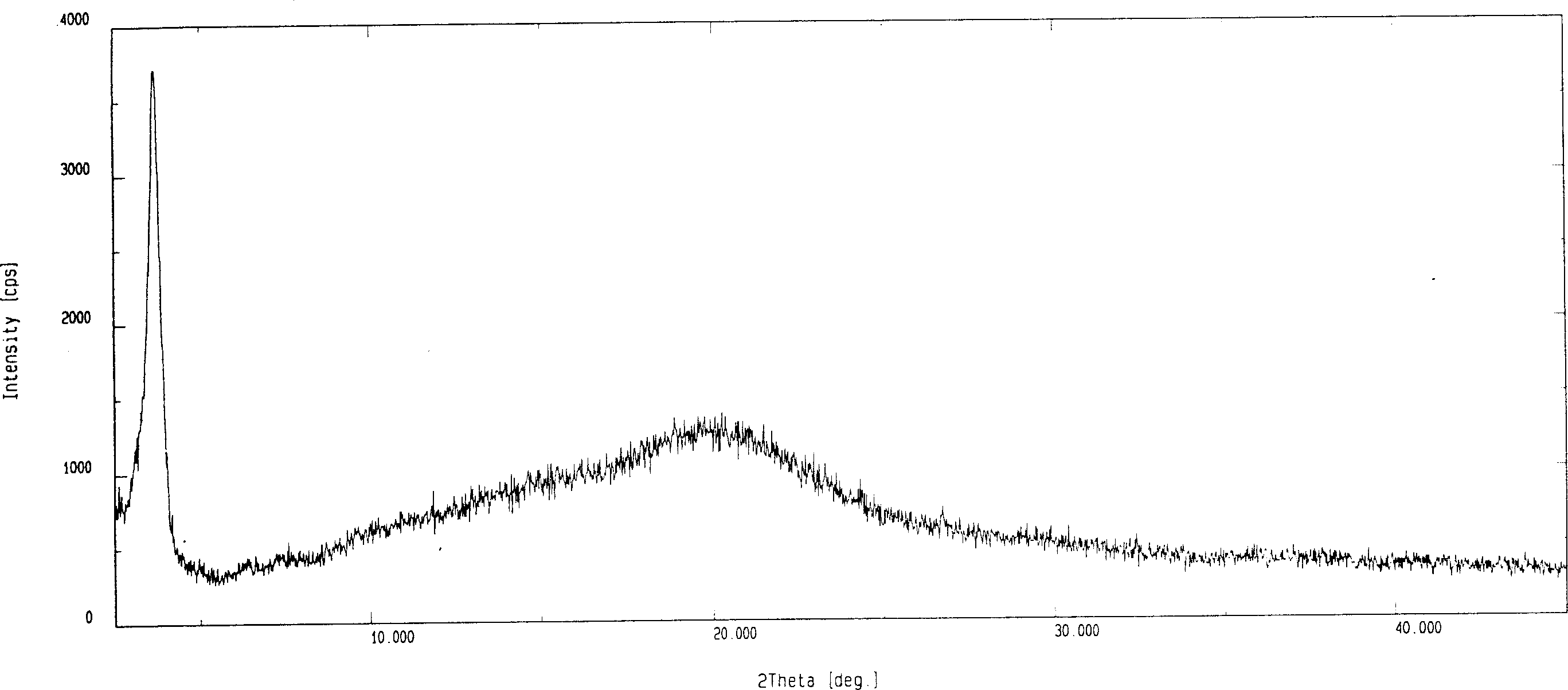
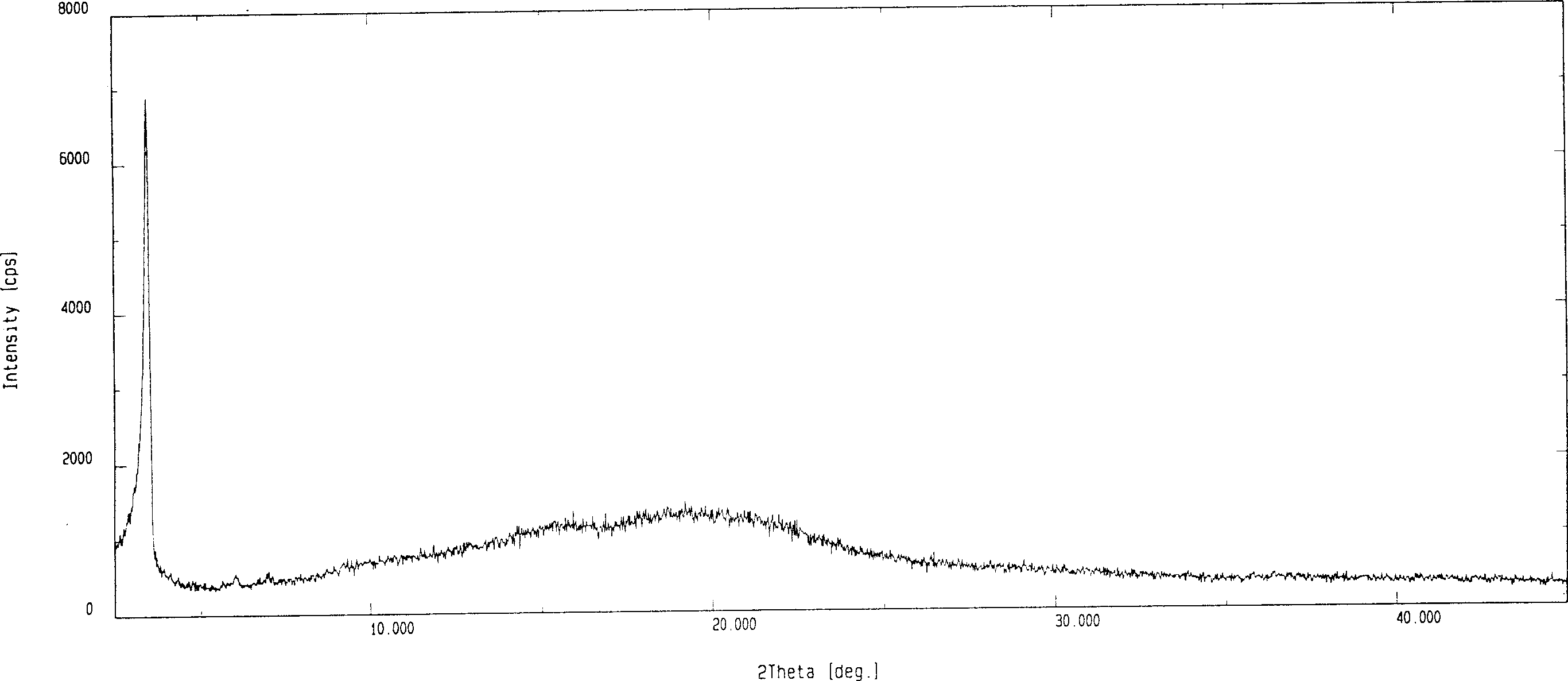
[0030] The X-ray diffraction figure of the amorphous fluvastatin sodium prepared by the present embodiment is as follows: image 3 As shown, it can be seen that fluvastatin sodium has reached the amorphous state.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com