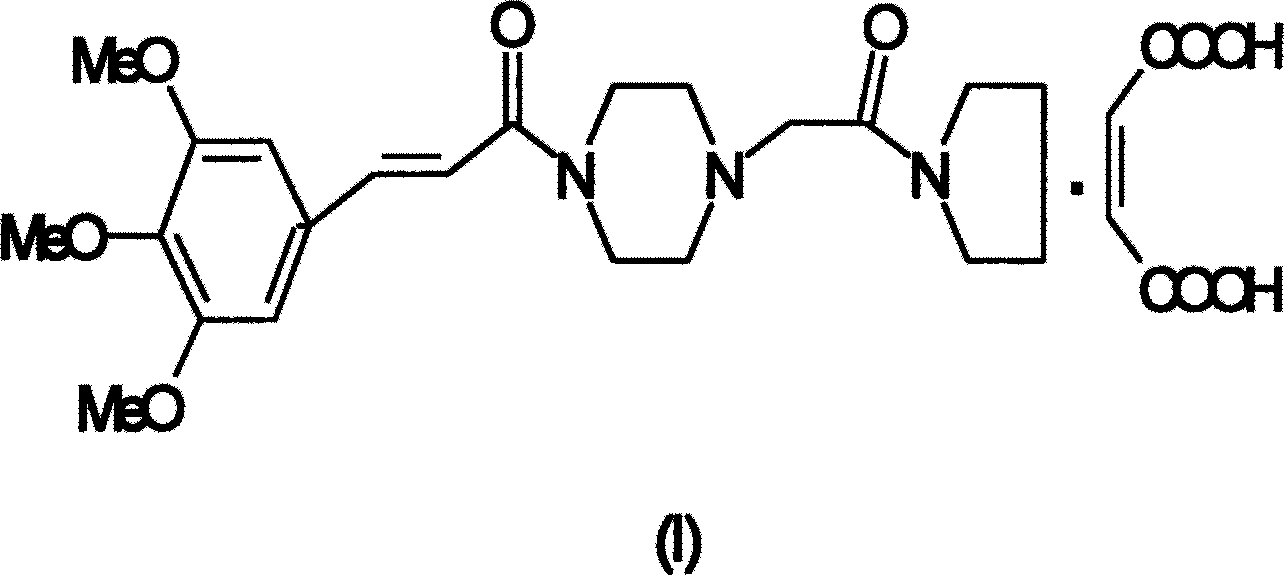
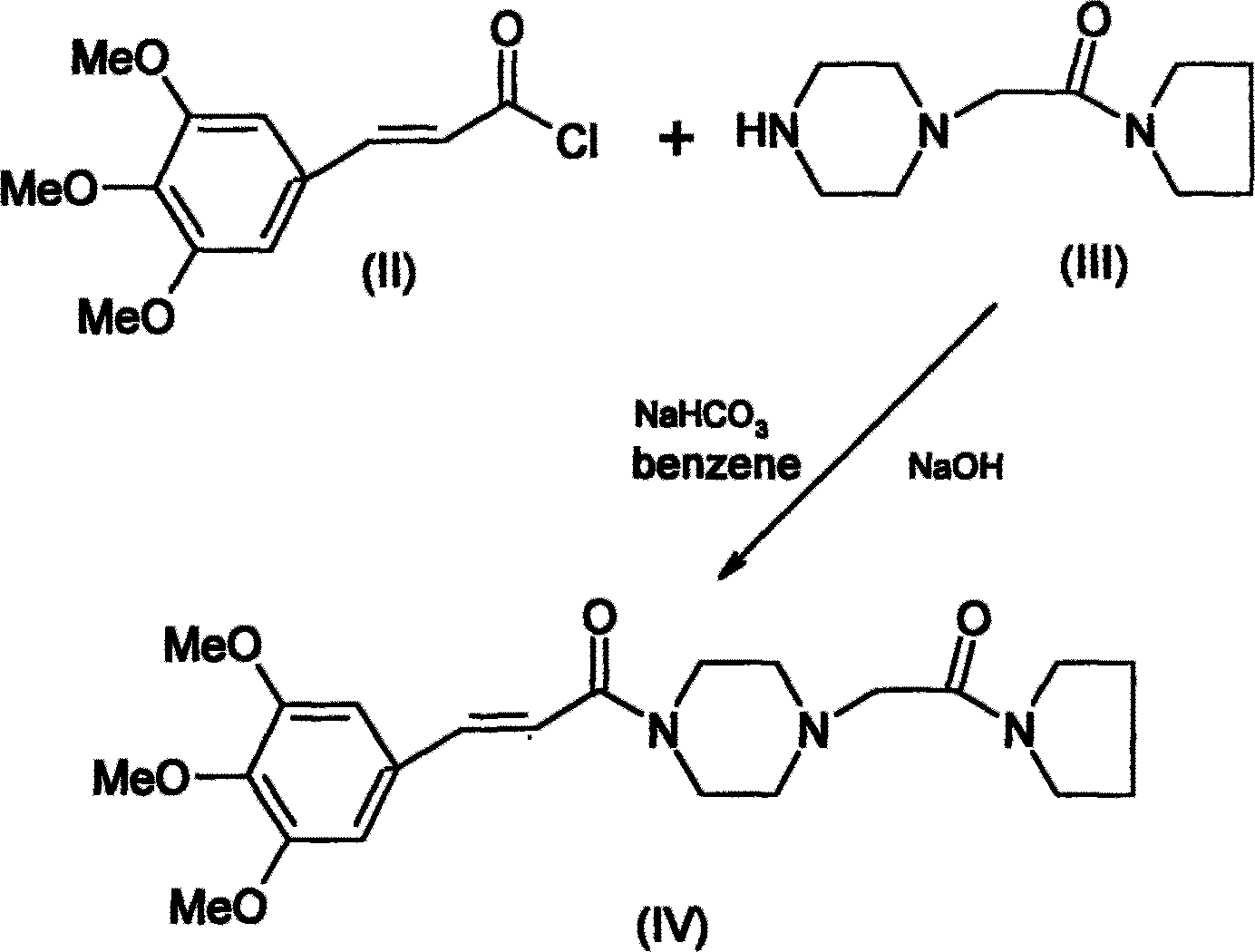
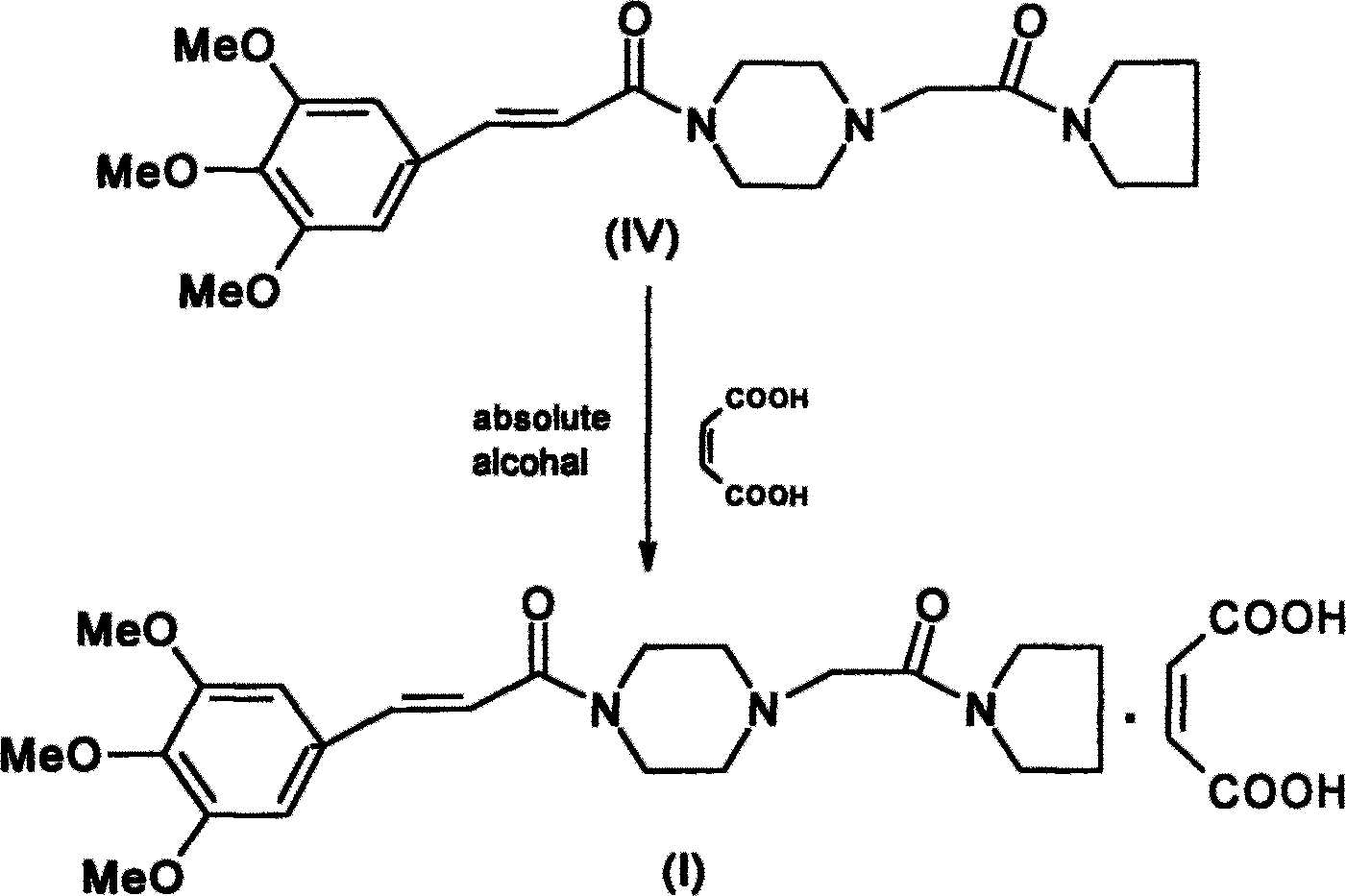
Improved cinepazide maleate preparation method
A technology of cinepazide maleate and piperazine, which is applied in the field of preparation of 1-[methyl]-4-piperazine maleate, and can solve the problems of large volume, low solubility and practical operation difficulties, etc. problem, to achieve the effect of high melting point and stable crystal shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Step 1, the preparation of chloroacetylpyrrolidine
[0054] Add 700 grams of chloroacetyl chloride into 2000 milliliters of chloroform, cool down to -10 to -5 °C under stirring, slowly add dropwise a solution of 440 grams of tetrahydropyrrole and 690 grams of triethylamine dissolved in 2000 milliliters of chloroform, and the addition is complete. Stir to return to room temperature, continue to react for 1 hour, add 300 ml × 3 water, wash and extract, dry over anhydrous sodium sulfate, evaporate the solvent to obtain a light green transparent liquid, solidify at room temperature, weighing 732 g, yield 80.2%. 1 HNMR (CDCl 3 ): δ1.85 (m, 2H, -CH 2 -), δ1.96(m, 2H, -CH 2 -), δ3.48[m, 4H, (-CH 2 -N) 2 ], δ3.99(s, 2H, O=C-CH 2 -Cl).
[0055] Step 2, the preparation of 1-[(1-tetrahydropyrrolecarbonyl)methyl]piperazine
[0056] Add 1020 grams of anhydrous potassium carbonate and 1830 grams of anhydrous piperazine into 4500 milliliters of absolute ethanol, stir and reflux...
Embodiment 2
[0067] Step 1, the preparation of chloroacetylpyrrolidine
[0068] Add 700 grams of chloroacetyl chloride into 2000 milliliters of chloroform, cool down to -10 to -5 °C under stirring, slowly add dropwise a solution of 440 grams of tetrahydropyrrole and 690 grams of triethylamine dissolved in 2000 milliliters of chloroform, and the addition is complete. Stir to return to room temperature, continue to react for 1 hour, wash and extract with 300 ml × 3 water, dry over anhydrous sodium sulfate, evaporate the solvent to obtain a light green transparent liquid, solidify at room temperature, weigh 732 g, yield 80.2%. 1 HNMR (CDCl 3 ): δ1.85 (m, 2H, -CH 2 -), δ1.96(m, 2H, -CH 2 -), δ3.48[m, 4H, (-CH 2 -N) 2 ], δ3.99(s, 2H, O=C-CH 2 -Cl).
[0069] Step 2, the preparation of 1-[(1-tetrahydropyrrolecarbonyl)methyl]piperazine
[0070] Add 1020 grams of anhydrous potassium carbonate and 1830 grams of anhydrous piperazine into 4500 milliliters of absolute ethanol, stir and reflux, add...
Embodiment 3
[0081] Step 1, the preparation of chloroacetylpyrrolidine
[0082] Add 700 grams of chloroacetyl chloride into 2000 milliliters of chloroform, cool down to -10 to -5 °C under stirring, slowly add dropwise a solution of 440 grams of tetrahydropyrrole and 690 grams of triethylamine dissolved in 2000 milliliters of chloroform, and the addition is complete. Stir to return to room temperature, continue to react for 1 hour, wash and extract with 300 ml × 3 water, dry over anhydrous sodium sulfate, evaporate the solvent to obtain a light green transparent liquid, solidify at room temperature, weigh 732 g, yield 80.2%. 1 HNMR (CDCl 3 ): δ1.85 (m, 2H, -CH 2 -), δ1.96(m, 2H, -CH 2 -), δ3.48[m, 4H, (-CH 2 -N) 2 ], δ3.99(s, 2H, O=C-CH 2 -Cl).
[0083] Step 2, the preparation of 1-[(1-tetrahydropyrrolecarbonyl)methyl]piperazine
[0084] Add 1020 grams of anhydrous potassium carbonate and 1830 grams of anhydrous piperazine into 4500 milliliters of absolute ethanol, stir and reflux, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com