Method for producing titanium oxide, photocatalytically active mixture, method for forming titanium oxide film utilizing them, method for producing article coated with titanium oxide and article coate
A film-forming method and technology of titanium oxide film, applied in the directions of titanium oxide/hydroxide, physical/chemical process catalyst, titanium compound, etc., can solve the cost of extra titanium oxide manufacturing, waste titanium oxide, and titanium oxide can not be photocatalyst function and other issues, to achieve the effect of seeking manufacturing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] (A) Production of samples
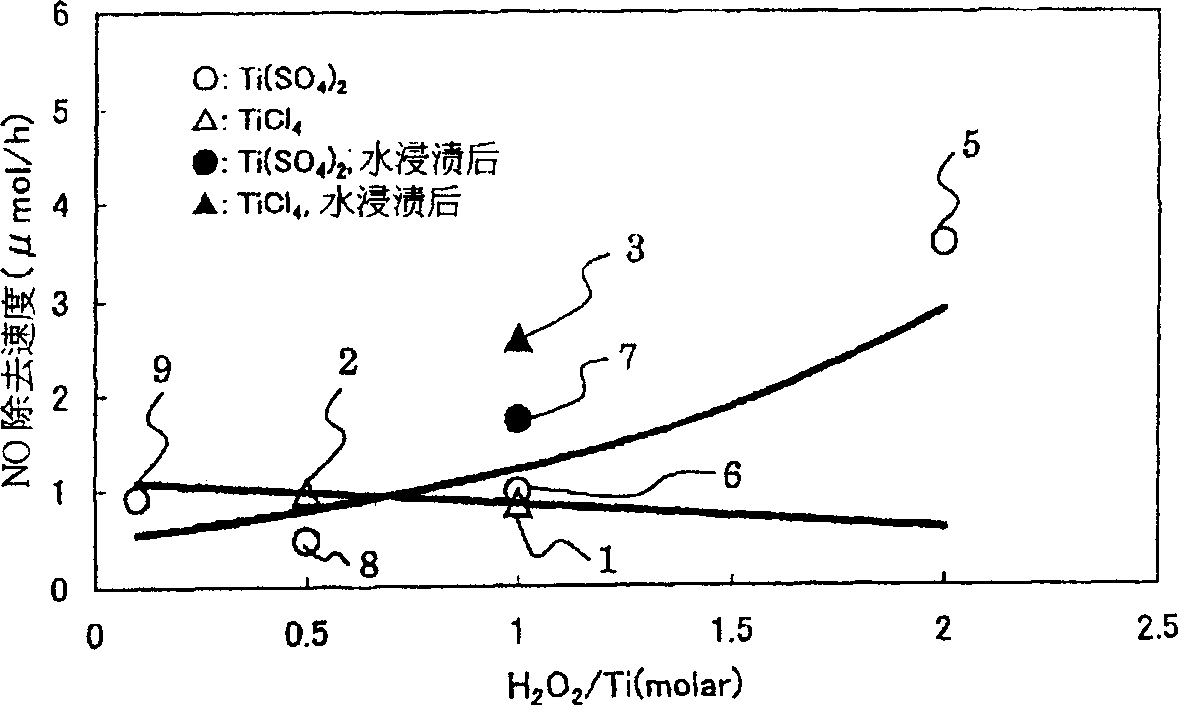
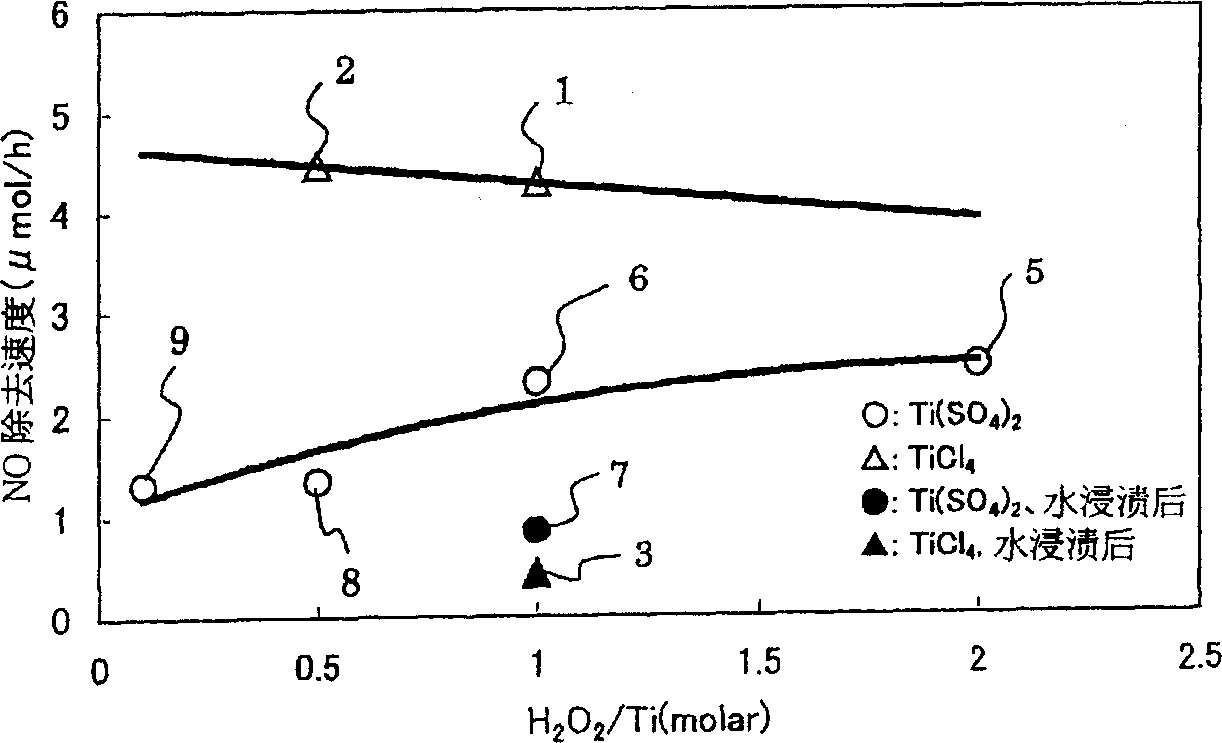
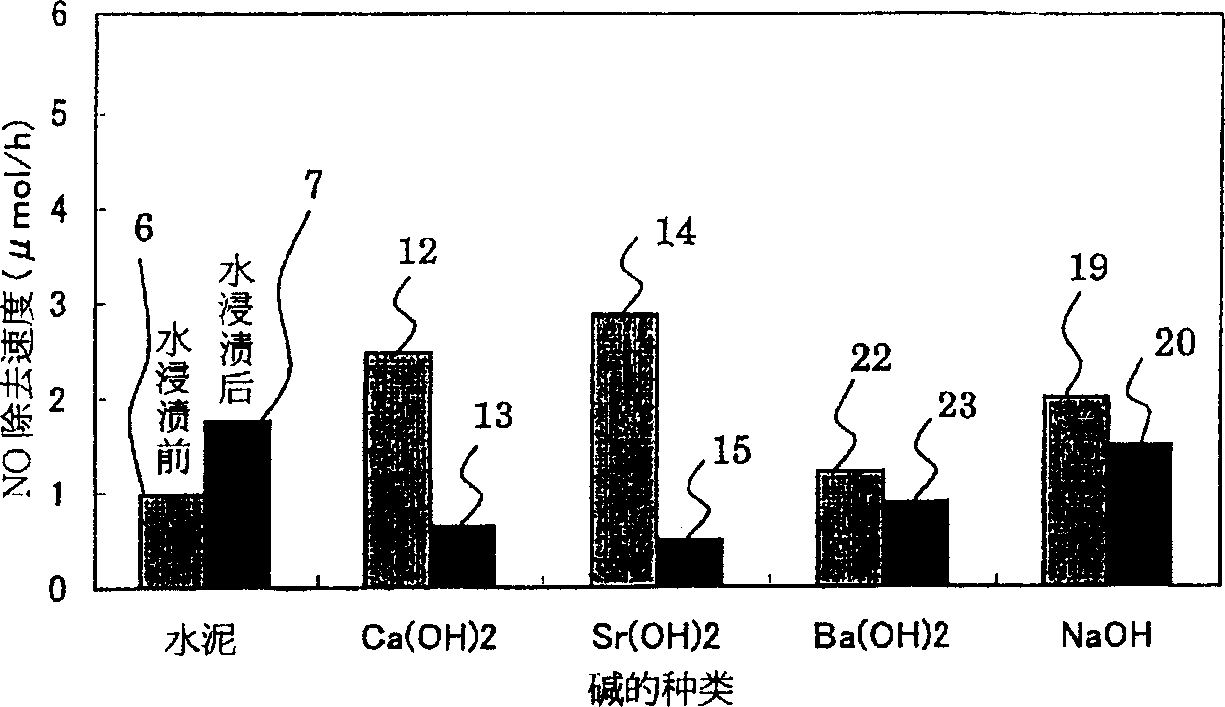
[0168] Samples 1 to 36 were manufactured by using Examples 1 to 5 and Comparative Examples 1 and 2 below. In addition, the concentrations of the respective aqueous solutions and spraying amounts in samples 1 to 36 are in accordance with Tables 1 to 3.
[0169] [Table 1]
[0170] Test No.
Alkali coating amount
Composition and spray amount of titanium-hydrogen peroxide aqueous solution
Number of impregnation / drying steps
NO adsorption speed
(μmol / h)
NO photooxidation rate
(μmol / h)
Alkali type
coating status
Coating amount
(g / 200cm 2 )
Titanium salt type
Titanium concentration
(wt%)
Hydrogen peroxide concentration
(wt%)
Spray amount
(g-solution / 200cm 2 )
1
pulp
10
TiCl 4
0.60
0.22
2.0
0
0.982
4.45
2
cement
pulp
10
TiCl 4
...
experiment example 2
[0184] Sample 16 and sample 17 were manufactured by the following procedure.
[0185] (1) The step of holding the alkali-containing material on the object to be filmed
[0186] A slurry-like alkali-containing material was applied to the surface of a concrete block (the same as in Experimental Example 1) as a film-forming object. As shown in Table 1, cement was used as the alkali-containing material.
[0187] (2) Step of attaching a soluble titanium salt solution to the surface of the object to be filmed
[0188] On the surface of the cement held by the concrete block, it is adhered by spraying an aqueous solution of a soluble titanium salt. Here, as the soluble titanium salt aqueous solution, a titanium sulfate aqueous solution is used.
[0189] (3) The step of attaching hydrogen peroxide to the object to be filmed
[0190] Adhesion is carried out by spraying hydrogen peroxide on the cement surface to which the titanium sulfate aqueous solution is attached.
[0191] (4) S...
experiment example 3
[0194] Sample 18 was produced through the following steps.
[0195] (1) The step of attaching the pertitanate ion aqueous solution to the object to be film-formed
[0196] Adhesion was carried out by spraying 10 g of pertitanate ion aqueous solution on the alkali-containing material which is the object of film formation. Here, as the alkali-containing material, 25 g of fly ash was used. This fly ash is an alkaline powder and has an alkali on its surface. In addition, as shown in Table 1, as the pertitanate ion aqueous solution, a mixed solution of titanium sulfate aqueous solution and hydrogen peroxide was used.
[0197] And, after spraying the pertitanate ion aqueous solution, it was dried.
[0198] (2) Steps of accumulating fly ash on concrete block
[0199] The fly ash to which the aqueous solution of pertitanate ions adhered was deposited on a concrete block (same as in Experimental Example 1).
[0200] (A-4) Experimental Example 4
[0201] Samples 19 to 29 were manu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com