Silioxane precursor containing azobenzene dye and its synthesis
A synthesis method, azobenzene technology, applied in the field of siloxane precursors and their synthesis, can solve the problems of rare design and synthesis research, difficult to achieve optical integration, easy to damage, etc., and achieve good second-order nonlinear optical properties , high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
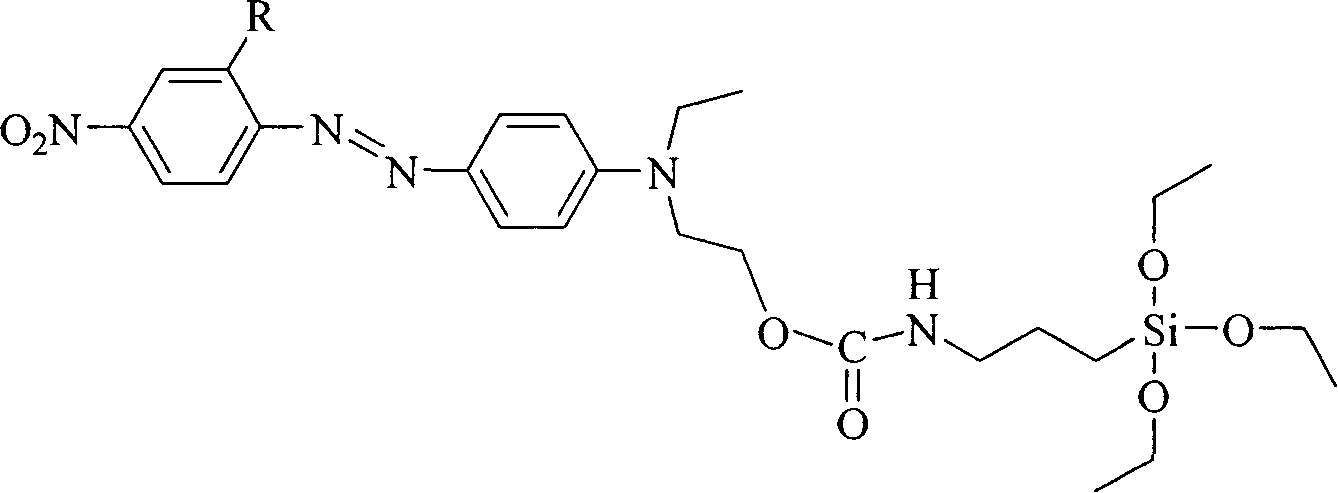
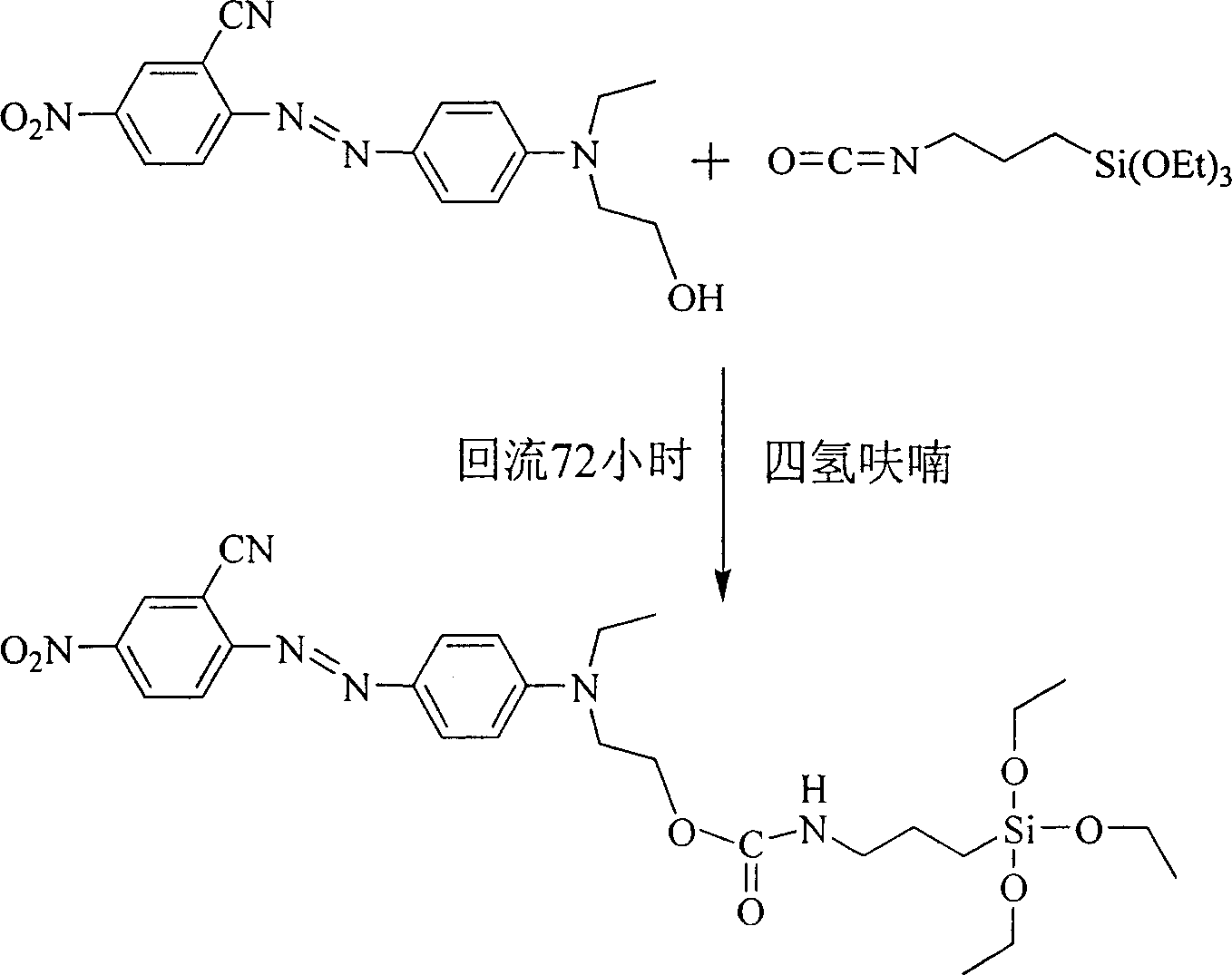
[0019] R in the general structural formula 1 is a cyano group. The synthetic route of the siloxane precursor containing azobenzene dye is as follows:
[0020]
[0021] The synthesis method is:
[0022] Add 10 mmol of 4'-(N-ethyl-N-hydroxyethyl)-amino-2-cyano-4-nitrate to a three-necked flask equipped with a reflux condenser and connected to an anhydrous and oxygen-free device at room temperature After opening the anhydrous and oxygen-free device and passing nitrogen gas for 30 minutes, add 20ml of freshly dried tetrahydrofuran and 3-5 drops of stannous octoate, stir magnetically, and then add 2.47g (10mmol) isocyanatopropane Triethoxysilane, heated to 90°C after the dropwise addition, refluxed for 72 hours, poured the reaction solution into n-hexane to produce a precipitate, filtered it with suction and dried it in vacuum to obtain a siloxane precursor containing azobenzene dye. Yield 73%, the second harmonic coefficient d after making the material 33 It is 46.1pm / V.
Embodiment 2
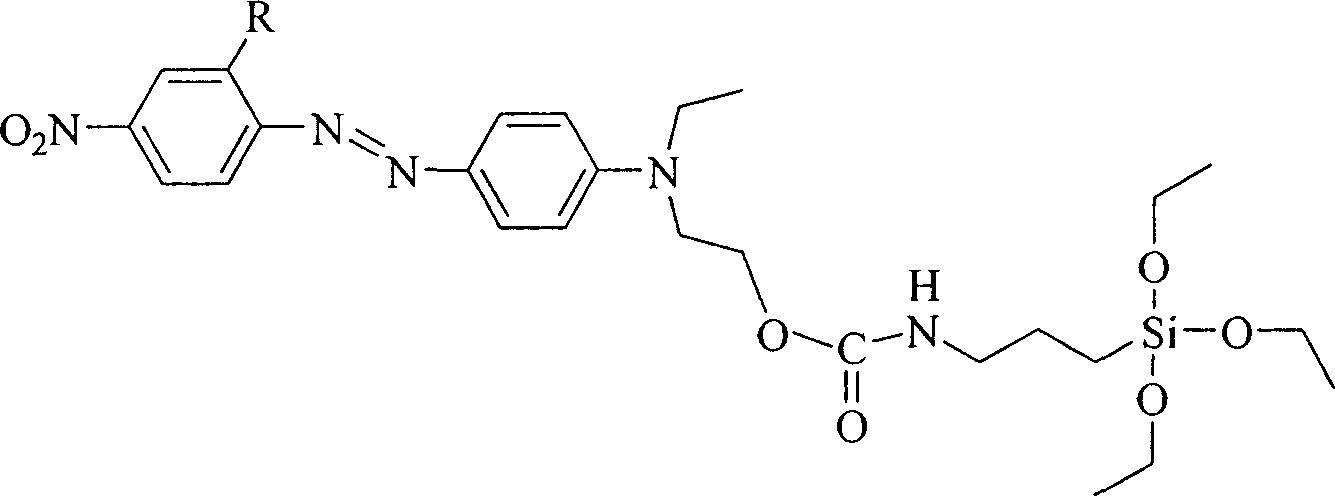
[0024] R in Structural Formula 1 is a nitro group. The synthetic route of the siloxane precursor containing azobenzene dye is as follows:
[0025]
[0026] The synthesis method is:
[0027] At room temperature, add 10 mmol of 4'-(N-ethyl-N-hydroxyethyl)-amino-2,4-dinitrobis to a three-necked flask equipped with a reflux condenser and connected to an anhydrous and oxygen-free device Azobenzene, after opening the anhydrous and oxygen-free device and passing nitrogen gas for 30 minutes, add 30ml of freshly dried acetone and 8-10 drops of triethylamine, stir magnetically, and then add 2.96g (12mmol) of isocyanatopropyl triethylamine Ethoxysilane, heated to 110°C after dropping, refluxed for 6 hours, poured the reaction solution into n-heptane to produce a precipitate, filtered it with suction and dried it in vacuum to obtain a siloxane precursor containing azobenzene dye. Yield 62%, the second harmonic coefficient d after making the material 33 It is 39.6pm / V.
Embodiment 3
[0029] R in Structural Formula 1 is a chlorine atom. The synthetic route of the siloxane precursor containing azobenzene dye is as follows:
[0030]
[0031] The synthesis method is:
[0032] Add 10 mmol of 4'-(N-ethyl-N-hydroxyethyl)-amino-2-chloro-4-nitro Azobenzene, after opening the anhydrous and oxygen-free device and passing nitrogen gas for 30 minutes, add 60ml of freshly dried dioxane and 5-6 drops of tin dodecyl laurate, magnetically stir, then add 4.94g (20mmol ) isocyanatopropyltriethoxysilane, heated to 140°C after the dropwise addition, and poured the reaction solution into petroleum ether to produce a precipitate after reflux for 48 hours, suction filtered and vacuum-dried to obtain silicon containing azobenzene dye Oxygen precursors. Yield 68%, the second harmonic coefficient d after making the material 33 It is 40.7pm / V.
[0033] The synthesis process of the siloxane precursor in the above examples is simple, the yield is high, and it has good second-or...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com