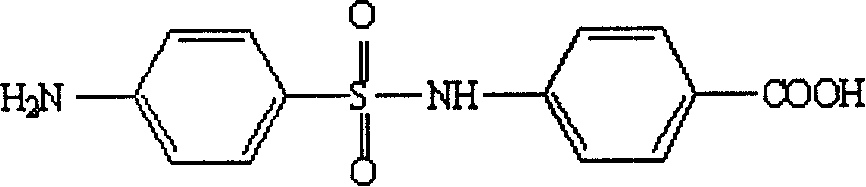
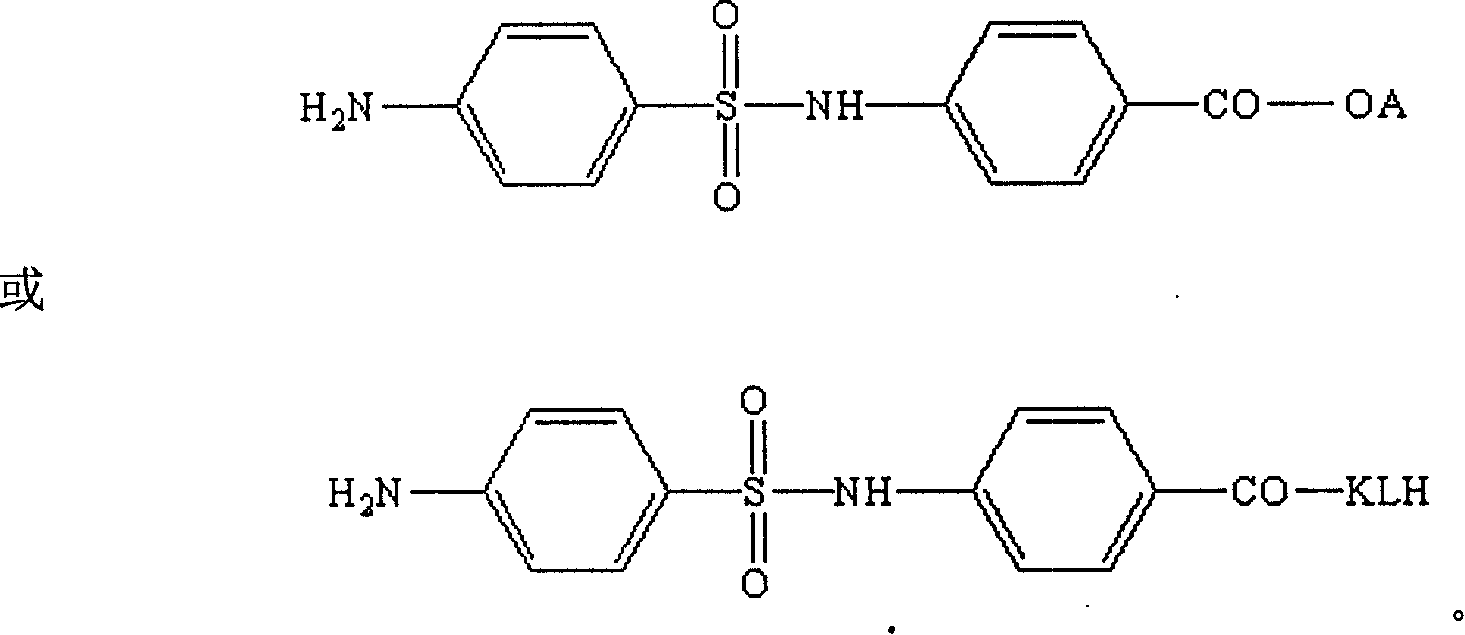
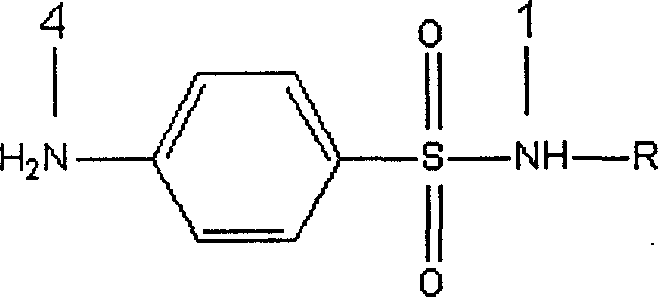
Artificial antigen used for immune analysis of sulfanilamide multi kind residue and antibody and its preparation
An immunoassay, artificial antigen technology, applied in analytical materials, material testing products, measuring devices, etc., to achieve the effect of easy popularization, simple synthesis method and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1. Hapten Synthesis
[0043] Dissolve 2 mmol of 4-acetylaminobenzenesulfonyl chloride in 5 mL of dry pyridine, N 2 Under protection, 6 mL of a dry pyridine solution containing 2 mmol of 4-aminobenzoic acid was added dropwise, and magnetically stirred. After the dropwise addition, the reaction mixture was refluxed at 90°C for 3 h, and the progress of the reaction was monitored by TLC, followed by the addition of 20 mL of 2 mol·L -1 NaOH, pyridine was removed under reduced pressure. The reaction system was refluxed for another 2 h, and the reaction progress was monitored by TLC, then cooled to 15°C, and the pH value was adjusted to 3.5 with concentrated hydrochloric acid. The product was extracted 3 times with ethyl acetate, 20 mL each time, the organic phases were combined and washed with anhydrous Na 2 SO 4 Dry and remove solvent under reduced pressure. Purify the residue through a 200-300 mesh silica gel column to obtain the target hapten 4-(4-aminobenzenesulfonyl...
Embodiment 2
[0054] 1. The specific method of hapten synthesis is:
[0055] 2.12 mmol of 4-aminobenzoic acid was dissolved in 6 mL of dry pyridine solution, 2.28 mmol of 4-acetylaminobenzenesulfonyl chloride was dissolved in 5 mL of dry pyridine, N 2 Under protection, it was added dropwise into the 4-aminobenzoic acid solution and stirred by magnetic force. After the dropwise addition, the reaction mixture was refluxed at 95°C for 2h, followed by the addition of 2mol L -1 NaOH 25mL, pyridine was removed under reduced pressure. The reaction system was refluxed for another 2.5 hours, cooled to 20°C, and the pH value was adjusted to 4.0 with concentrated hydrochloric acid. The product was extracted with ethyl acetate (3×30 mL), the organic phases were combined and washed with anhydrous Na 2 SO 4 Dry and remove solvent under reduced pressure. The residue was purified through a silica gel column (200-300 mesh) to obtain the target product;
[0056] 2. The specific method of artificial ant...
Embodiment 3
[0064] 1. Hapten Synthesis
[0065] Dissolve 3.5 mmol 4-acetylaminobenzenesulfonyl chloride in 5 mL dry pyridine, N 2 Under protection, 6 mL of a dry pyridine solution containing 3 mmol of 4-aminobenzoic acid was added dropwise, and magnetically stirred. After the dropwise addition, the reaction mixture was refluxed at 85°C for 3 h, and the progress of the reaction was monitored by TLC, followed by the addition of 30 mL of 2 mol L -1 NaOH, pyridine was removed under reduced pressure. The reaction system was refluxed for another 3 h, and the reaction progress was monitored by TLC, then cooled to 25° C., and the pH value was adjusted to 4.5 with concentrated hydrochloric acid. The product was extracted 3 times with ethyl acetate, 40 mL each time, the organic phases were combined and washed with anhydrous Na 2 SO 4 Dry and remove solvent under reduced pressure. Purify the residue through a 200-300 mesh silica gel column to obtain the target hapten 4-(4-aminobenzenesulfonyl)b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dilution degree | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com