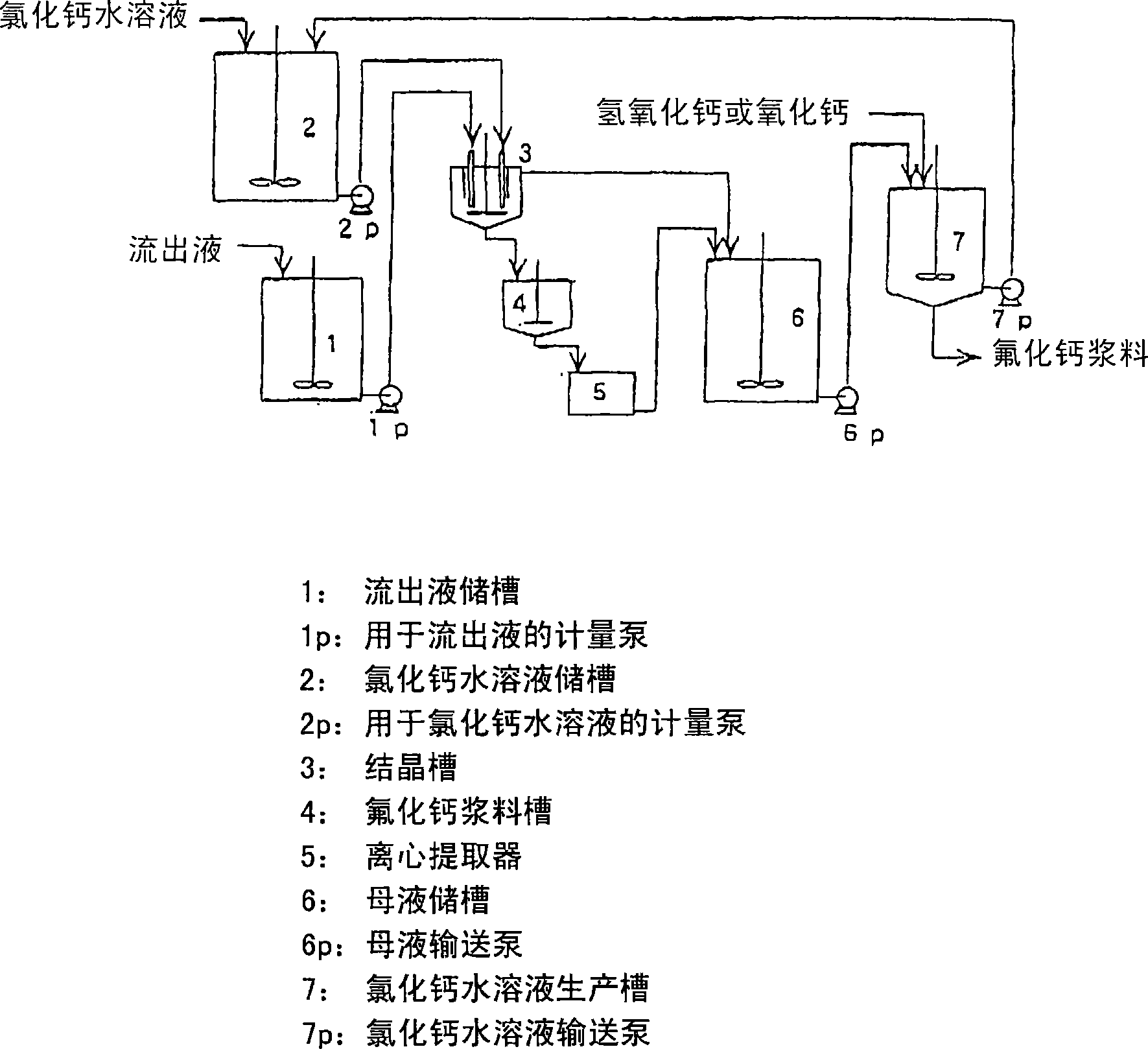
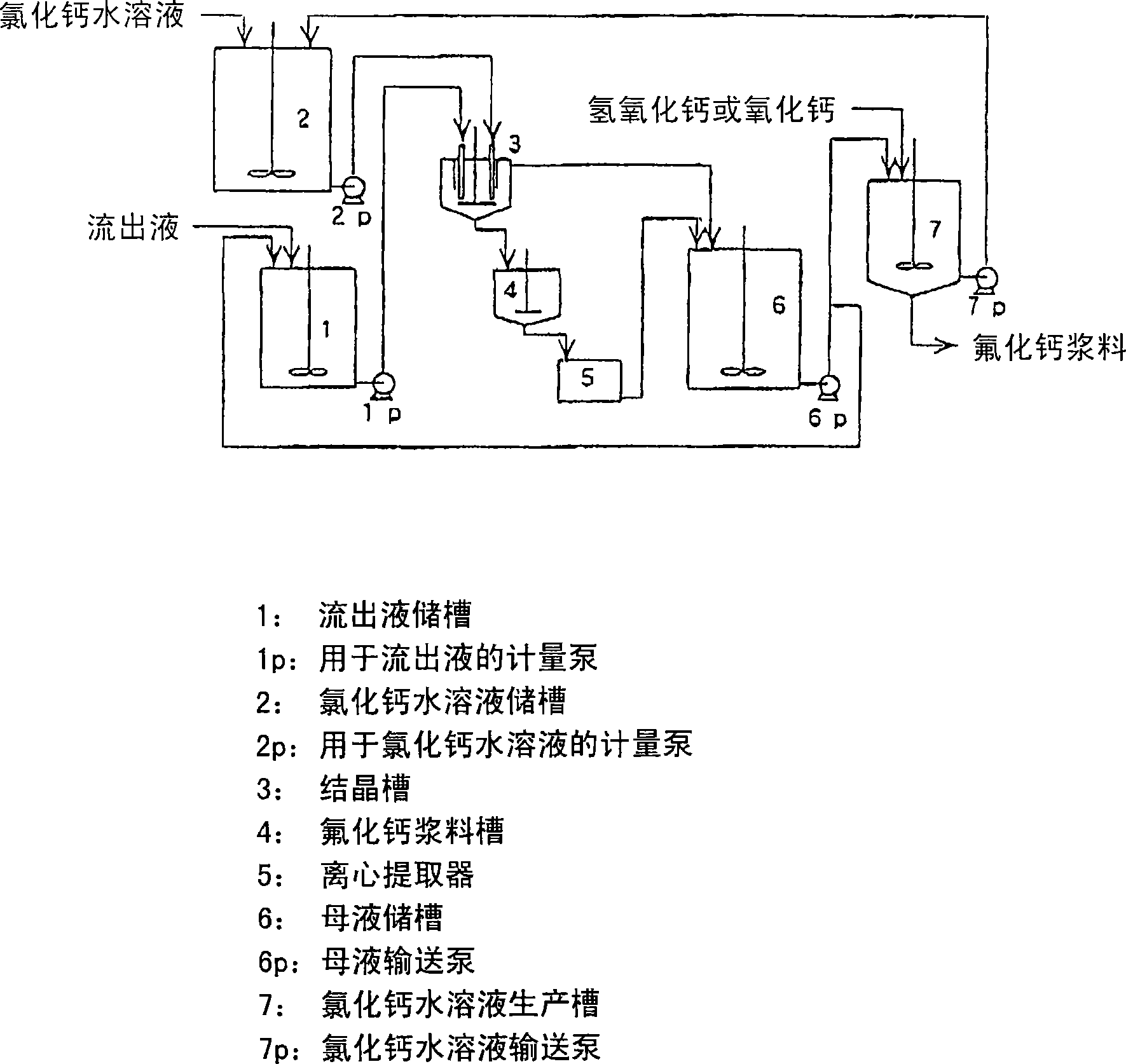
Method for producing calcium fluoride, reusing method and recycling method thereof
A technology for calcium fluoride and calcium fluoride production, which is applied in the field of recycling calcium fluoride and recycling calcium fluoride, and can solve the problems of increasing chlorine impurities, excessive secondary aggregation, and decreased hydrogen fluoride yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] 5 liters of hydrochloric acid of 1000 grams of 5% were housed in it was made by Teflon (registered trademark, hereinafter the same) PFA and a reactor equipped with a Teflon stirrer was placed in a water bath whose temperature was set at 50° C., and within 5 hours, Preferably under the balance of the metering pump, drop 1200 grams of 1000 grams of fluoride-containing neutral effluent (containing 21.1% potassium fluoride, 7.2% potassium chloride and 0.4% fluorine) into the stirred solution. NaCl) (totally 7.1% fluoride) and 200 grams of 35% hydrochloric acid mixed solution, drop into 1482 grams of 14% calcium chloride aqueous solution simultaneously. 10 minutes after the dripping was completed, the stirring was stopped, the solution was subjected to suction filtration, and 3520 g of filtrate was recovered. The residue was washed slightly and then dried at 120° C. for 2 hours to recover 134.2 g of calcium fluoride (92% recovery). The purity of calcium fluoride is 99.1%, t...
Embodiment 2
[0081] A 5 liter reactor made of Teflon PFA and equipped with a Teflon stirrer containing 1000 grams of 2% hydrochloric acid is placed in a water bath set at 50° C. and within 5 hours, preferably under metering pump equilibrium, In this stirring solution, drop 1100 grams of a mixed solution consisting of 1000 grams of pH of 9 fluoride-containing alkaline effluent (containing 2.4% sodium fluoride) and 100 grams of 35% hydrochloric acid. 1610 grams of 2% calcium chloride in water. 10 minutes after the dripping was completed, the stirring was stopped, the solution was subjected to suction filtration, and 3650 g of filtrate was recovered. The residue was washed slightly and then dried at 120° C. for 2 hours to recover 15.0 g of calcium fluoride (67% recovery). The purity of calcium fluoride is 99.0%, the average particle size is 9.8 microns, and the loss after burning at 500°C for two hours is 0.23%. The mother liquor contains 1.5% hydrochloric acid and 0.2% calcium fluoride. ...
Embodiment 3
[0083] A 5 liter reactor made of Teflon PFA and equipped with a Teflon stirrer containing 1000 g of 15% hydrochloric acid is placed in a water bath set at 50° C. and within 5 hours, preferably under metering pump equilibrium, 1000 grams of hydrofluoric acid-containing effluent (containing 17.2% hydrofluoric acid and 15.0% hydrochloric acid) was added dropwise to the stirred solution, and 1704 grams of 28% calcium chloride aqueous solution was added dropwise. 10 minutes after the dripping was completed, the stirring was stopped, the solution was subjected to suction filtration, and 3400 g of filtrate was recovered. The residue was washed slightly and then dried at 120° C. for 2 hours to recover 287.6 g of calcium fluoride (86% recovery). The purity of calcium fluoride is 99.3%, the average particle size is 27.3 microns, and the loss after burning at 500°C for two hours is 0.23%. The mother liquor contains 17.9% hydrochloric acid and 1.4% calcium fluoride. mother liquor pH ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com