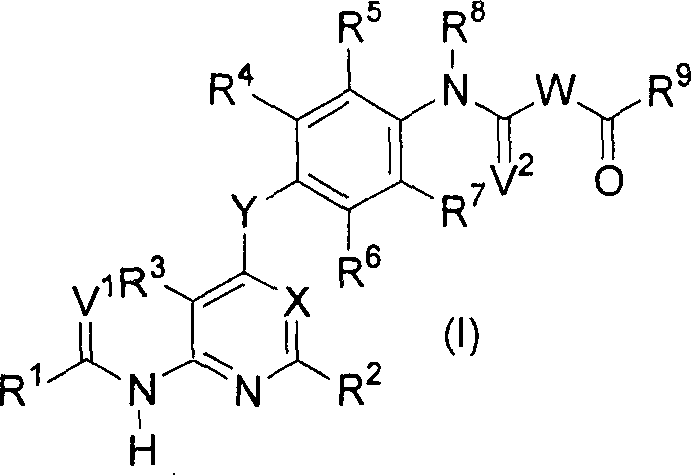
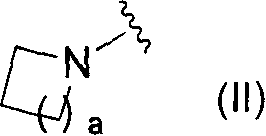
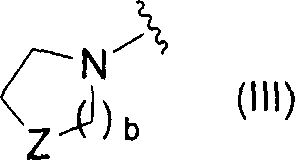Novel pyridine derivative and pyrimidine derivative (1)
A technology of compounds and hydrates, which is applied in the direction of drug combinations, active ingredients of heterocyclic compounds, cardiovascular system diseases, etc., and can solve problems such as unrecorded compounds and undisclosed HGFR inhibitory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0324] This step is a step of obtaining compound (1t) from compound (1ps) (compound (1ps) refers to compound (1p) and compound (1s) described in [Production method 1-B]). The same method as can be used.
[0325]
[0326] This step is a step of obtaining compound (1u) from compound (1t). The same method as can be used.
[0327]
[0328] This step is the protection group " R of compound (1u) 2 positions 102 The step of deprotecting -O-C(=O)-" and "P" to obtain compound (1n). Depending on the type of protecting group, deprotection using an acid such as hydrochloric acid or trifluoroacetic acid, using sodium hydroxide, Deprotection reaction of inorganic base such as potassium hydroxide, deprotection reaction using tetrabutylammonium fluoride, etc., deprotection reaction of catalytic hydrogenation reaction using palladium carbon, palladium hydroxide, etc. as catalyst, etc., to obtain compound (1n) .
[0329]
[0330] In this step, only the protecting groups "R" at the t...
Embodiment
[0586] The compounds of the present invention can be produced, for example, by the methods described in the following Preparations and Examples. However, the following examples are illustrative only, and the compounds of the present invention are not limited to the following specific examples in any way.
[0587] Unless otherwise specified in the preparation examples and examples, YMC SIL-60-400 / 230W is used to represent silica gel for purification.
[0588] In addition, as the LC-MS purification conditions, unless otherwise specified, the conditions described below were employed.
[0589] ODS column (WakopakR Combi ODS Column, or YMC Combi ODS-A)
[0590] Solvent A liquid 0.1% trifluoroacetic acid-water, B liquid 0.1% trifluoroacetic acid-acetonitrile
[0591] Flow rate 30mL / min
[0592] retention time 10min
[0593] gradient
[0594] 0.00min A: 99%, B: 1%
[0595] 8.00min A: 20%, B: 80%
[0596] 8.20min A: 0%, B: 100%
preparation example 1
[0597] (Preparation example 1) 0.5M hexane solution of phenylacetyl isooxyacid
[0598] In a nitrogen atmosphere, oxalyl chloride (3.51 mL, 40.2 mmol) was added to a suspension of phenylacetamide (1.81 g, 13.4 mmol) in 1,2-dichloroethane (150 mL) at room temperature, and stirred at 110° C. one night. After cooling the reaction solution to room temperature, it was concentrated under reduced pressure, n-hexane (26.8 mL) was added thereto, and ultrasonic treatment was performed. The supernatant (yellow solution fraction) was used as the title reagent for subsequent reactions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com