Amphipathic fluorescence target nano micelle and its preparation method
A nanomicelle, amphiphilic technology, applied in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problems such as the inability to effectively introduce active end groups and the complex process of synthesizing carriers. , to achieve good biocompatibility and degradability, long-term safe drug release, and uniform distribution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Preparation of PLA-PEI amphiphilic carrier
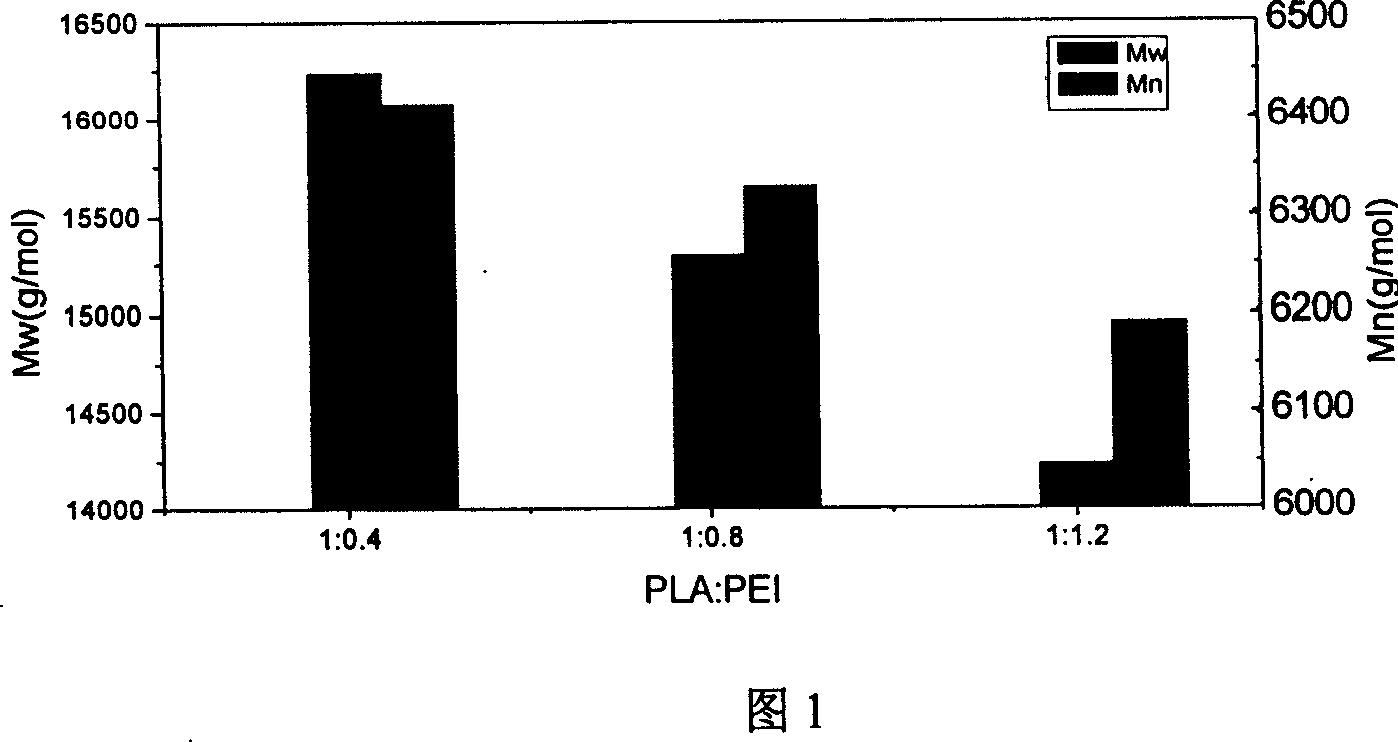
[0040] (1) PLA:PEI=1:0.4
[0041] Get PLA, N, N-dicyclohexylcarboimide (DCC), N-hydroxysuccinimide (NHS) are dissolved in dichloromethane (DCM), stir at room temperature for 24hr, prepare activated PLA; Take activated PLA Dissolve 1g of PLA in 10ml of DCM (A), 0.0121g of PEI in 5ml of acetone (B); after mixing evenly, slowly add system B to system A with high-speed stirring, and perform aminolysis for 6hr; add the obtained solution to high-speed stirring Precipitate in ice-cold ether; Buchner funnel filtration, vacuum drying to obtain white PLA-PEI powder.
[0042] The obtained PLA-PEI had Mn=6447 g / mol and Mw=16076 g / mol. (See Attachment 1)
[0043] (2) PLA:PEI=1:0.8
[0044] Dissolve PLA, DCC, and NHS in DCM and stir at room temperature for 24 hours to prepare activated PLA; take 1g of activated PLA and dissolve in 10ml of DCM (A), and dissolve 0.0242g of PEI in 5ml of acetone (B); after mixing them unifor...
Embodiment 2
[0049] Embodiment 2: preparation PLA-PEI is the drug-loaded micelle of carrier
[0050] Dissolve 0.5g PLA-PEI in 20ml DCM, dissolve evenly and become an oil phase, and ultrasonically suspend 5-Fu powder in the oil phase; dissolve 1g Tweeen-80 in 100ml water to become a water phase; Add it dropwise into the water phase, continue to stir for 48 hours until the organic solvent evaporates, put it in the refrigerator for 24 hours to further solidify the micelles; centrifuge, rinse with triple distilled water three times, and freeze-dry to obtain nano drug-loaded micelles powder.
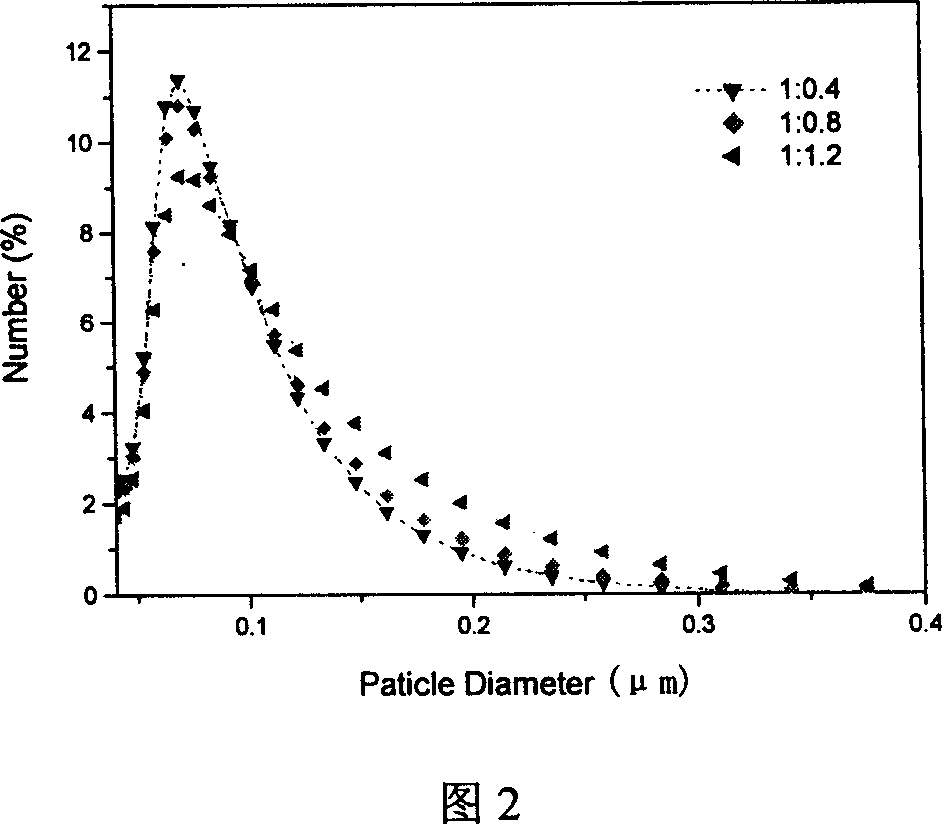
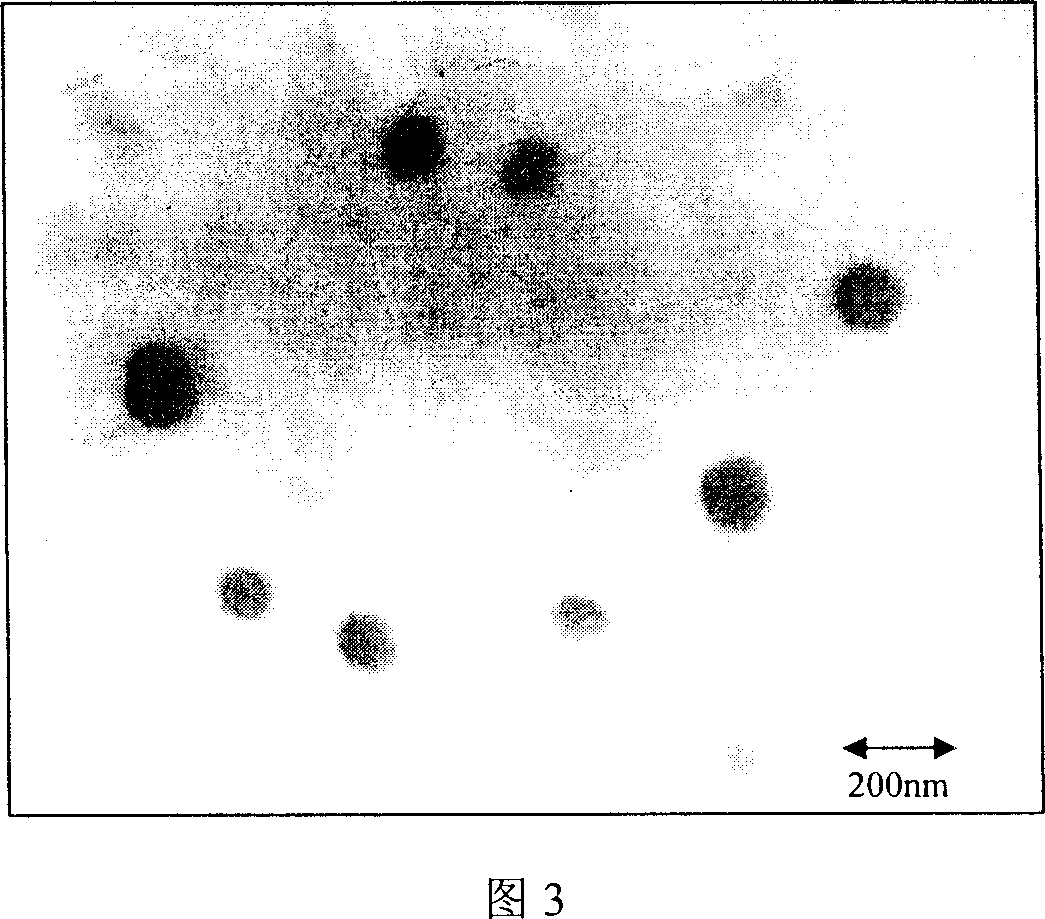
[0051] The obtained nanometer drug-loaded micelles have a particle diameter of about 100nm (see accompanying drawing 2), are rounded and spherical, and have uniform sizes. (See Attachment 3)
[0052] The standard curve equation of 5-Fu at 265nm in 0.1M dilute hydrochloric acid is:
[0053] A=0.0567563C+0.000651 (r=0.9999) C is the concentration of 5-Fu (μg / ml), and A is the absorbance.
[0054] ...
Embodiment 3
[0055] Example 3 Preparation of Amphiphilic Targeted Nanomicelles
[0056] The drug-loaded micelles with PLA-PEI as the carrier were suspended in a certain amount of phosphate buffer solution (PBS) with a pH value of 4.0 (C); 0.026g folate and 0.0226g EDAC were dissolved in 5ml DMSO (D ), under magnetic stirring for 3hr; slowly drop system C into D system, keep for 10hr under magnetic stirring, adjust with PBS solution with a pH value of 4.0, and always keep the pH value of the system at 5.0; centrifuge the micellar suspension, three Rinse with distilled water for three times, and freeze-dry for 24 hours to obtain powder targeting nanomicelles.
[0057] Weigh a certain amount of targeting nanomicelle, dissolve it in DMSO, measure the absorbance at 362nm, and substitute it into the standard curve equation:
[0058] PLA: PEI
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com