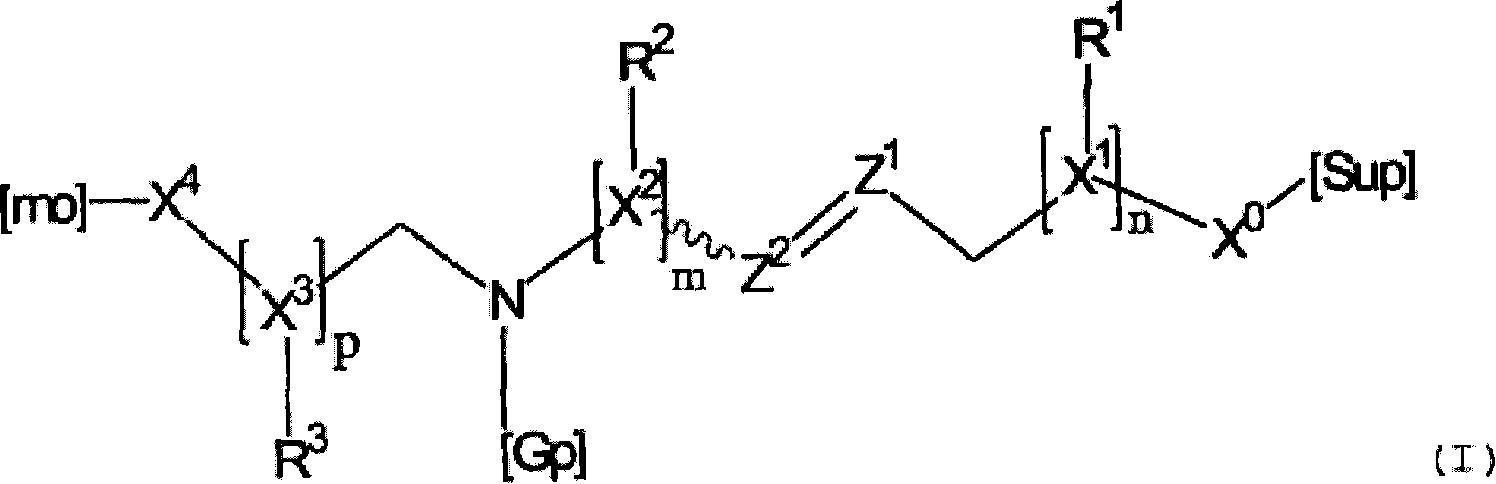
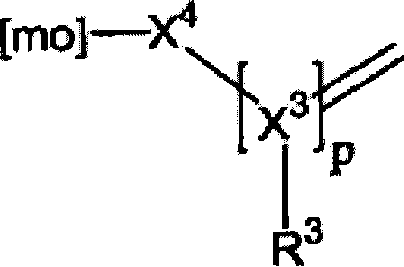
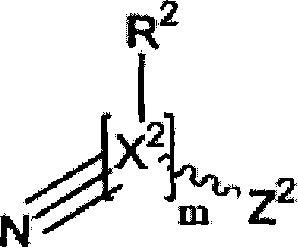Molecular spacer, production method thereof and uses of same on an analysis chip with molecules or biomolecules
A technology of biomolecules and spacer arms, applied to biochips and microsystems for bioanalysis, biochips, application fields on analysis chips
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0099] Example A: Activation of oligosaccharides (Reaction A)
[0100] In this example, an ozone decomposition reaction was used. The method used is described in [5].
[0101] The sugar (1) (0.93 mmol) allylated at the anomeric position was dissolved in a mixture of 5 ml of dichloromethane and methanol (1 / 1): the medium was immersed in a cold bath (acetone + Dry ice). Then the ozone O 3 Bubbling into the solution: As soon as blue (characteristic of excess ozone) appears, replace ozone with argon (or nitrogen). When the reaction is complete, by adding dimethyl sulfide Me 2 S (4.65mmol, 5eq) reduced the medium: DMSO was then formed. After overnight, the medium slowly returned to ambient temperature and then evaporated under vacuum: the organic residue was diethyl ether Et 2 O was extracted and then washed with water. The organic phase was evaporated under vacuum and then co-evaporated with toluene. The crude product was purified by silica gel column chromatography (eluent: petroleum...
Embodiment B
[0103] Example B: Reduction of Nitriles (Reaction B)
[0104] The method used is described in [6].
[0105] LiAlH 4 (381mg, 10.03mmol, 1eq) was added to freshly distilled diethyl ether (20ml).
[0106] 4-Pentenenitrile (3) (814 mg, 1 ml, 10.03 mmol) was slowly added to the reaction medium with a temperature of 0° C. (ice bath) and stirring under a nitrogen atmosphere. The stirring must be continued for approximately 20 minutes at ambient temperature.
[0107] Next, add water (0.4ml), then a 20% aqueous sodium hydroxide solution (0.3ml), and finally another amount of water (1.4ml): these additions must be carried out with great care, because the neutralization effect may be severe . When the diethyl ether solution is separated from the inorganic white residue by precipitation, the supernatant is extracted.
[0108] The white solid (residue) was washed twice with diethyl ether, and the organic phases were combined. A 3M hydrochloric acid HCl solution was added to the organic phase i...
Embodiment C
[0115] Example C: Reductive amination (Reaction C)
[0116] The chemical method used is described in [7].
[0117] The aldehyde (2) (20.87mmol) is dissolved in the calcium hydride (CaH 2 ) In freshly distilled dimethylformamide (1.2ml): stir the medium and add amine (4) (31.30mmol, 2eq). After about 20 minutes, add sodium cyanoborohydride NaBH 3 CN (83.47 mmol, 4 eq) was added to the mixture, and the mixture was continuously stirred at ambient temperature overnight.
[0118] If the reaction is not complete, you can add NaBH 3 CN(1eq). Then, when the reaction was completed, pyridine (2.4 ml) and acetic anhydride (83.47 mmol, 2 eq / amine) were added to the mixture.
[0119] When the reaction was complete (about 1 hour after the addition), the crude compound was extracted with diethyl ether and water. Use magnesium sulfate (MgSO 4 ) Dry, then filter, evaporate under vacuum, and then co-evaporate with toluene.
[0120] The compound (5) was then purified by silica gel chromatography (el...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com