Pharmaceutical formulations for the sustained release of interleukins and therapeutic applications thereof
A sustained-release technology for interleukin, applied in the pharmaceutical formulation of sustained-release interleukin and its therapeutic applications, can solve problems such as loss of biological activity, denaturation, and protein contact, and achieve good local tolerance, Extended release time, good and stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0261] Example 1: Amphiphilic Polymer P1
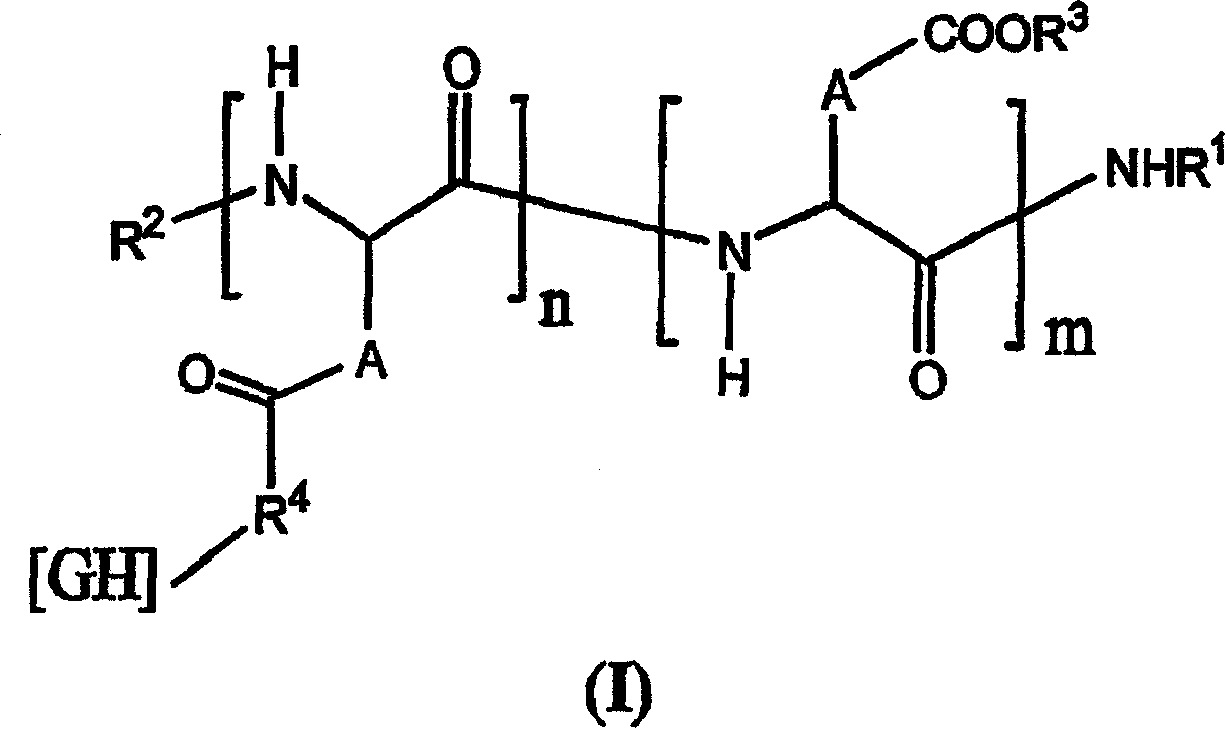
[0262] Synthesis of Linked Polyglutamic Acid and Synthetic α-Vitamins
[0263] 5.5 g of α-L-polyglutamic acid (molecular weight of about 10,000 daltons, obtained by hydrolysis and polymerization of NCAGIuOMe relative to standardized polyoxyethylene, refer to patent application FR-a-2801226). Heat at 40°C for two hours and dissolve in 92 ml of dimethylformamide (DMF). After the polymer was dissolved, the temperature was lowered to 25° C., and 1.49 g D, L-alpha-vitamin (>98%, Fluka®) pre-dissolved in 6 ml DMF, 0.09 g 4-dimethylamino Pyridine and 0.57 g of diisopropylcarbodiimide predissolved in 6 ml of DMF. After stirring at 25° C. for 8 hours, the reaction system was poured into 800 ml of water containing 15% sodium chloride and hydrochloric acid (pH 2). The polymer precipitate was then filtered, washed with 0.1N hydrochloric acid and then water and recovered. The polymer was then redissolved in 75 ml DMF and reprecipitated in pH 2...
Embodiment 2
[0264] Example 2: Amphiphilic polymers P1, P2, P3, P4, P5 and P6
[0265] These polymers were prepared in the same manner as polymer P1. Table 1 below summarizes the properties of these polymers. The properties of polymer P1 are also given for comparison.
[0266] polymer
Molecular weight of polyglutamic acid
1 g / mol
Hydrophobic
% Connectivity (NMR) 2
polymer molecule
Amount 1g / mol
P1
10000
alpha-vitamins 3
7
13900
P2
10000
alpha-vitamins 3
4
14400
P3
16900
alpha-vitamins 3
4
15200
P4
10000
5
l1500
P5
16900
5
12900
P6
10000
15
11500
[0267] 1 equivalent to polyoxyethylene
[0268] 2 Molecular attachment rates assessed by proton NMR
[0269] 3 synthetic source
Embodiment 3
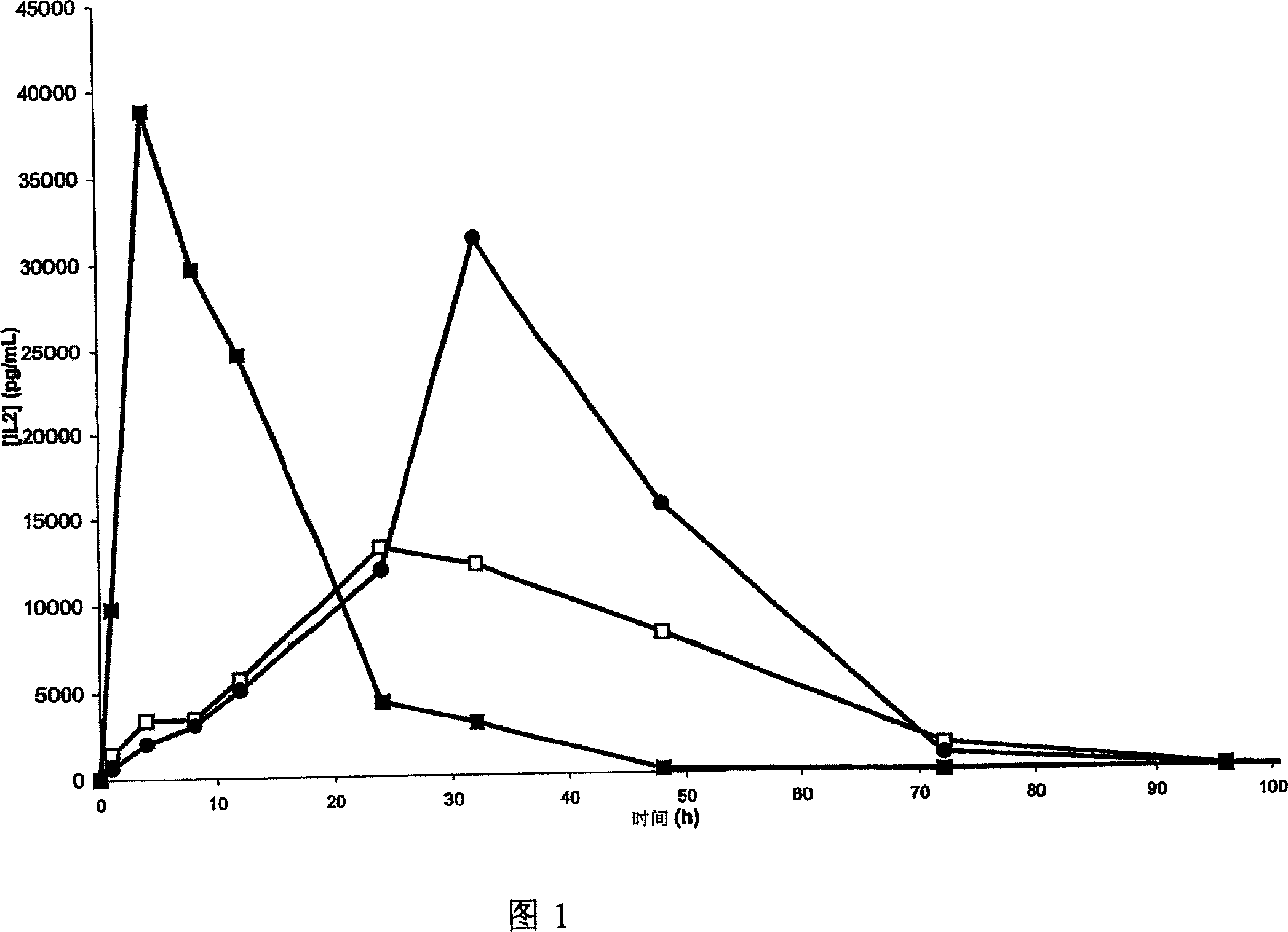
[0270] Example 3: Preparation of a long-acting interleukin 2 (IL2) formulation of the invention based on polymer P3
[0271] Sufficient amphiphilic polymer lyophilized powder and sterile water were placed in a flask to obtain a polymer concentration X that was 1.3 times the desired final concentration. The polymer was dissolved with magnetic stirring for 16 hours.
[0272] The required amount of lyophilized IL2 was concentrated to X / (X-1) times the desired final concentration.
[0273] The precise concentration of the concentrated IL2 solution was determined by a Perkin Elmer Lambda 35 UV spectrophotometer at 280 nm.
[0274] The IL2 solution was filtered through a 0.8-0.2 micron filter and stored at 4°C. The pH of the solution was adjusted to 11 with 1M NaOH. The ratio of the protein concentration of this solution to the concentration required by the recipe is called Y.
[0275] The protein solution and polymer solution were then mixed at room temperature, adding X-1 lite...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com