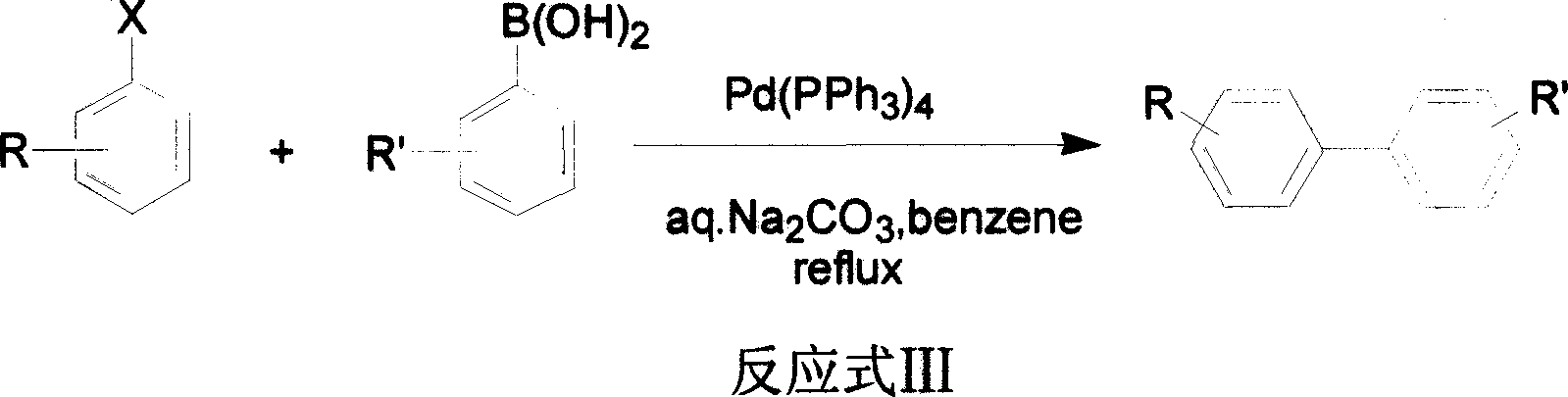
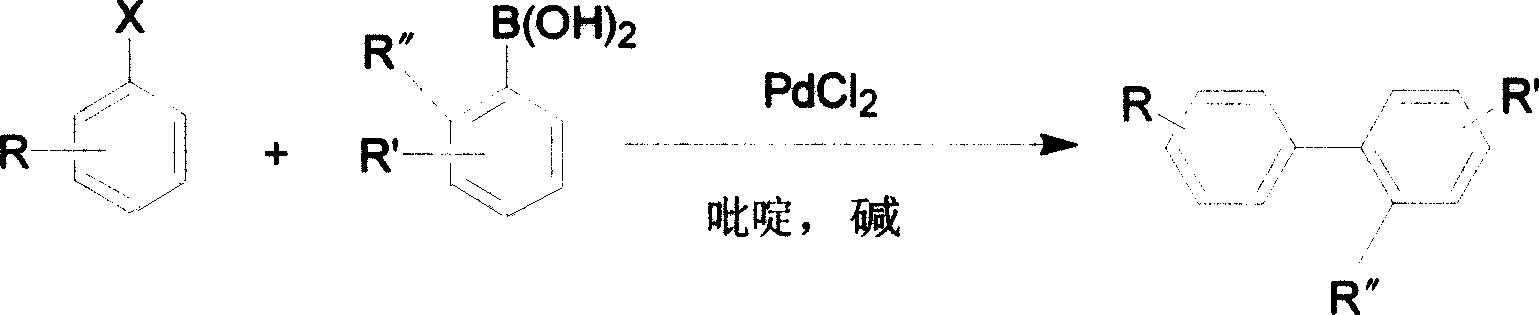
Method for cross coupling reaction utilizing substituted halogenated arene and substituted aryl boric acid
A cross-coupling reaction, halogenated aromatic hydrocarbon technology, applied in the direction of organic chemistry methods, chemical instruments and methods, carboxylic acid nitrile preparation, etc. Reduce the activity and other problems, to achieve the effect of large industrial practical value, low cost and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Synthesis of 4-(4-propylcyclohexyl)-4'-cyano-1,1'-biphenyl
[0027]
[0028] In the 50mL reaction flask, add 0.364g (2mmol) p-cyanobromobenzene, 0.738g (3mmol) 4-(4-propylcyclohexyl) phenylboronic acid (as number 1 in Table 1), 0.553g (4mmol) potassium carbonate, 1.7mg (0.01mmol) of palladium chloride and 4mL of pyridine; under the protection of nitrogen, the oil bath was heated to 115°C and refluxed for 5 hours. TLC showed that the bromide disappeared. After recovering the solvent under reduced pressure, the residue was separated by silica gel column chromatography (petroleum ether was the eluent) and recrystallized to obtain 0.515 g of colorless flaky crystals with a yield of 85%. Temperature (°C): Cr.133N>210.
[0029] 1 HNMR (CDCl 3 , 500MHz) δ=0.91(t, J=7.2Hz, 3H), 1.03~1.11(m, 2H), 1.21~1.25(m, 2H), 1.29~1.38(m, 3H), 1.45~1.53(m, 2H), 1.85~1.96(m, 4H), 2.48~2.57(m, 1H), 7.33(d, J=8.2Hz, 2H), 7.52(d, J=8.2Hz, 2H), 7.66~7.72(m , 4H).
Embodiment 2
[0030] Example 2 Synthesis of 4-(4-pentylcyclohexyl)-4'-cyano-1,1'-biphenyl
[0031]
[0032] In the 50mL reaction flask, add 0.364g (2mmol) p-cyanobromobenzene, 0.822g (3mmol) 4-(4-pentylcyclohexyl) phenylboronic acid (as Table 1 number 2), 0.553g (4mmol) potassium carbonate, 1.7mg (0.01mmol) of palladium chloride and 4mL of pyridine; under the protection of nitrogen, the oil bath was heated to 115°C and refluxed for 6 hours. TLC showed that the bromide disappeared. After recovering the solvent under reduced pressure, the residue was separated by silica gel column chromatography (petroleum ether was the eluent), and recrystallized to obtain 0.593 g of colorless flaky crystals with a yield of 87%. Temperature (°C): Cr.96 N>210.
[0033] 1 HNMR (CDCl 3 , 500MHz) δ=0.90(t, J=7.1Hz, 3H), 1.02~1.11(m, 2H), 1.21~1.36(m, 9H), 1.44~1.53(m, 2H), 1.85~1.97(m, 4H), 2.48~2.57(m, 1H), 7.32(d, J=8.3Hz, 2H), 7.52(d, J=8.1Hz, 2H), 7.66~7.71(m, 4H).
Embodiment 3
[0034] Example 3 Synthesis of 4-(4-propylcyclohexyl)-4'-(4-pentylcyclohexyl)-1,1'-biphenyl
[0035]
[0036] Add 0.562g (2mmol) 4-(4-propylcyclohexyl) bromobenzene to 50mL reaction flask, 0.822g (3mmol) 4-(4-pentylcyclohexyl) phenylboronic acid (as in Table 1 number 3), 0.553 g (4mmol) potassium carbonate, 1.7mg (0.01mmol) palladium chloride and 4mL pyridine; under the protection of nitrogen, the oil bath was heated to 115°C and refluxed for 5 hours, TLC showed that the bromide disappeared. After recovering the solvent under reduced pressure, the residue was separated by silica gel column chromatography (petroleum ether was the eluent) and recrystallized to obtain 0.533 g of colorless needle crystals with a yield of 62%. The purity was 99.5% as detected by HPLC, and the phase transition Temperature (°C): Cr.53S>210.
[0037] 1 HNMR (CDCl 3, 500MHz) δ=0.89~0.92(m, 6H), 1.05~1.10(m, 4H), 1.21~1.38(m, 14H), 1.44~1.52(m, 4H), 1.87~1.94(m, 8H), 2.48~2.54 (m, 2H), 7.24~7.28 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com