Levo lactone hydrolase producing fungus, and its method for preparing chiral hydroxy acid
A hydrolase and hydroxy acid technology, applied in the field of highly selective L-lactone hydrolase producing bacteria, can solve the problems of low substrate concentration, affecting the application effect, and insufficient optical purity of the product, and achieve stable enzyme production and stereoselectivity Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The fermentation culture of embodiment 1 Fusarium proliferatum ECU2002
[0041] Slant and plate medium (g / L): glycerol 30, yeast extract 7.5, peptone 7.5, agar 20. Sterilize at 121°C for 15 minutes, cool down after sterilization, plate, inoculate, and incubate at 30°C for 2 days. Fermentation medium (g / L): glycerol 30; peptone 10; yeast extract 10; NH 4 NO 3 3; Inorganic salt (g / L) (NaCl1; MgSO 4 ·7H 2 O1; FeSO 4 ·7H 2 O0.02; ZnSO 4 ·7H 2 O0.03; CuSO 4 ·5H 2 00.005); pH7.5. Sterilized at 121 DEG C for 15 minutes, cooled and inoculated after sterilization, with an inoculum size of 2%, fermented at 30 DEG C, under the condition of a rotating speed of 160r / min, and cultivated for 2 days when the dry weight of the thalline reached 18g / L, the enzyme production can reach more than 90U / L, and the specific activity is 5U / g.
Embodiment 2
[0042] The immobilization of embodiment 2 cell debris
[0043] 1) Take 50g of Fusarium proliferatum ECU2002 cells, add 5g of quartz sand to grind for 1h to break the cell wall, centrifuge at 12,000rpm for 15min, the supernatant is cell-free extract, and the precipitate is cell debris.
[0044] 2) Take 5 g of cell fragments, add different carriers, measure the viability after immobilizing the cell fragments, and measure their residual viability after storing at 4° C. for 1 week (results are shown in Table 1).
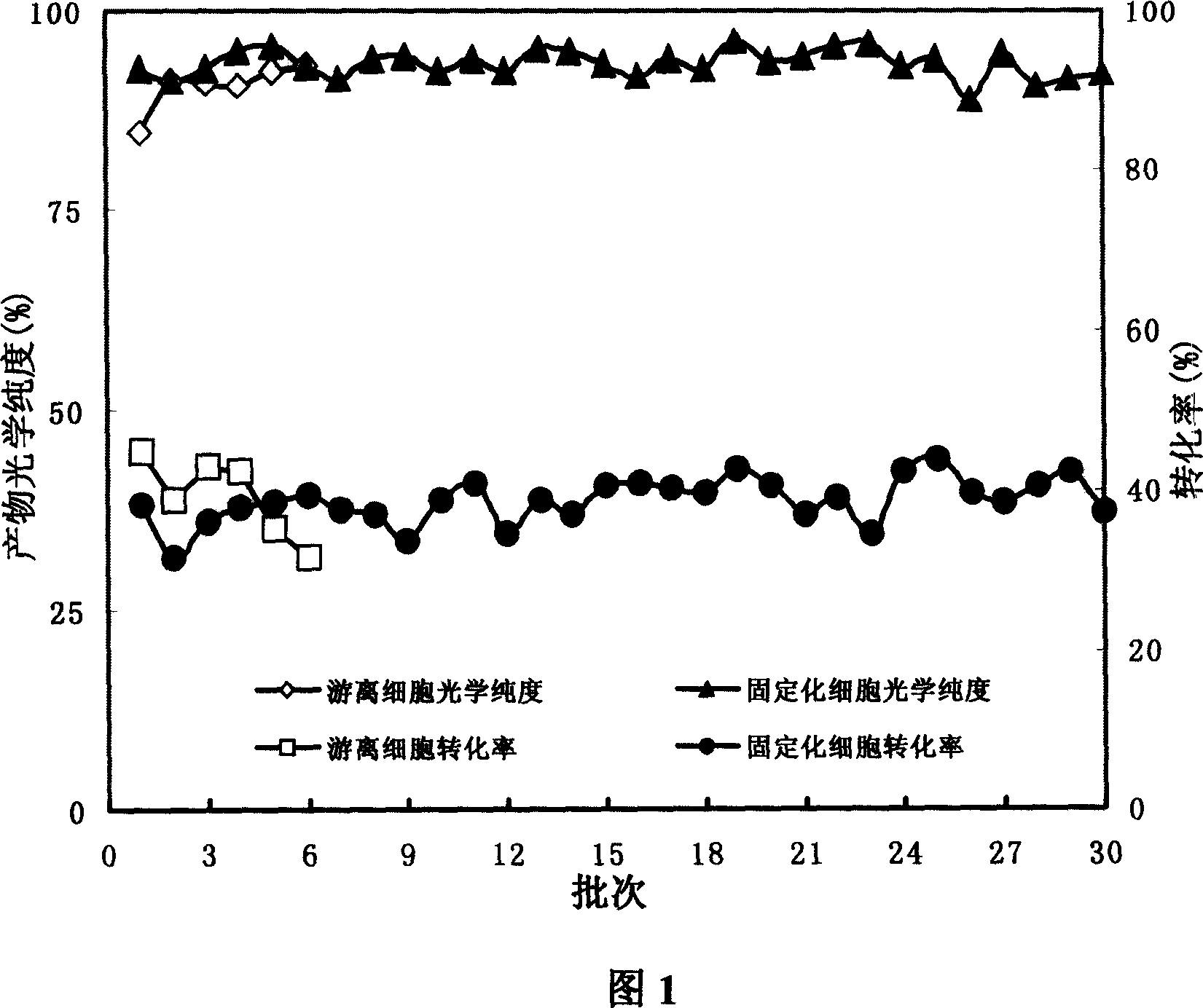
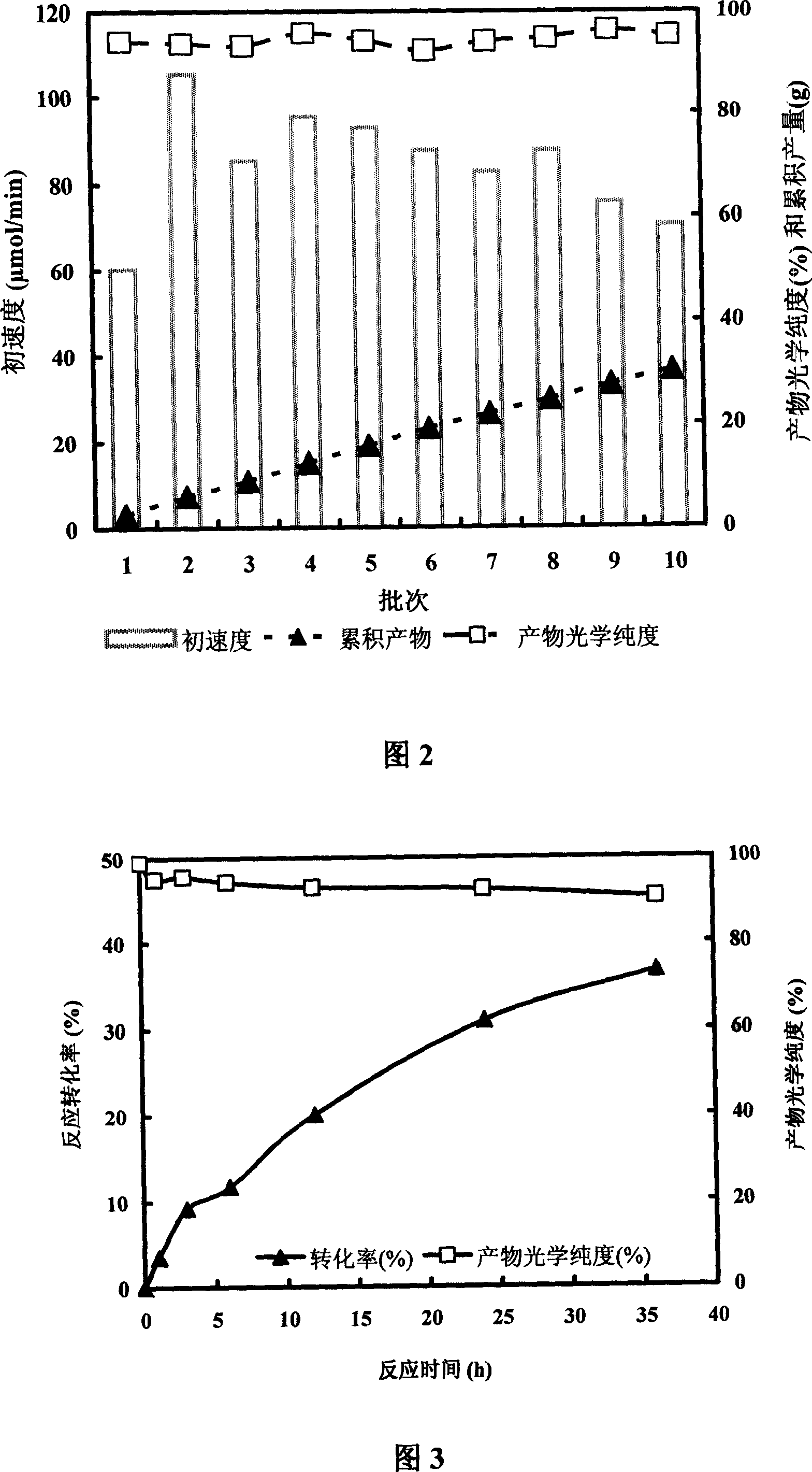
[0045] 3) Use glutaraldehyde-immobilized cell fragments as the enzyme source, (±)-pantolactone as the substrate, the reaction volume is 20mL, the substrate concentration is 2M, 10g of immobilized cell fragments are added, and the reaction temperature is 30°C. 3M ammonia water was added dropwise to maintain the pH of the reaction solution at 7.0. After a reaction time of 24 hours, the conversion rate was 34.4%, and the optical purity of the product (-)-pantolactone was 94...
Embodiment 3~13
[0048] Example 3-13 The hydrolysis activity of Fusarium proliferatum ECU2002 immobilized cells to a series of lactone compounds
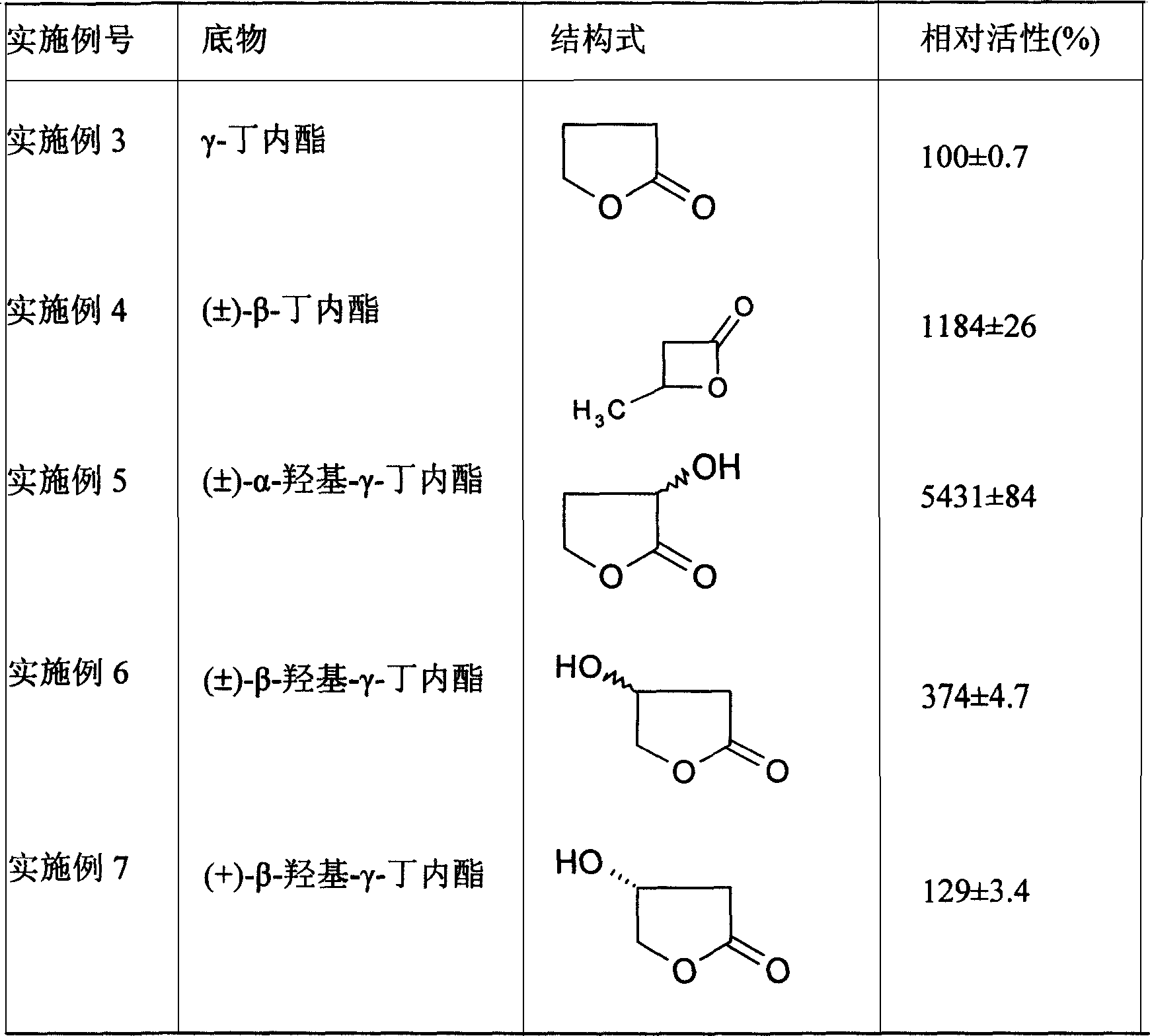
[0049] A series of lactones in the following list 2 are substrates, and the substrate concentration is 100 mM , the reaction volume was 10 mL, and 0.2 g of immobilized cells (cells obtained by cross-linking with 15 mM glutaraldehyde at 30° C. for 3 h) were added, and the viability was measured after reacting at 30° C. for 0.1 to 0.5 h under magnetic stirring. Table 2 lists the relative viability of immobilized cells when different lactones are hydrolyzed. Taking the activity of γ-butyrolactone as 100%, the hydrolysis effect of immobilized cells on lactones with relatively complex structures (such as n-butylphenyl peptide) is not obvious, and the activity of the enzyme is only 25%. Immobilized cells showed high activity to α-substituted lactone, and when the substrate was α-hydroxy-γ-butyrolactone, the activity was the highest, which was 54 times th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com