Non noble metal catalyst for cathode of direct methanol fuel cell, and preparation method
A methanol fuel cell and non-precious metal technology, applied in the direction of catalyst activation/preparation, battery electrodes, catalyst carriers, etc., can solve problems such as unstable electrocatalysts, affecting battery performance, and expensive prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
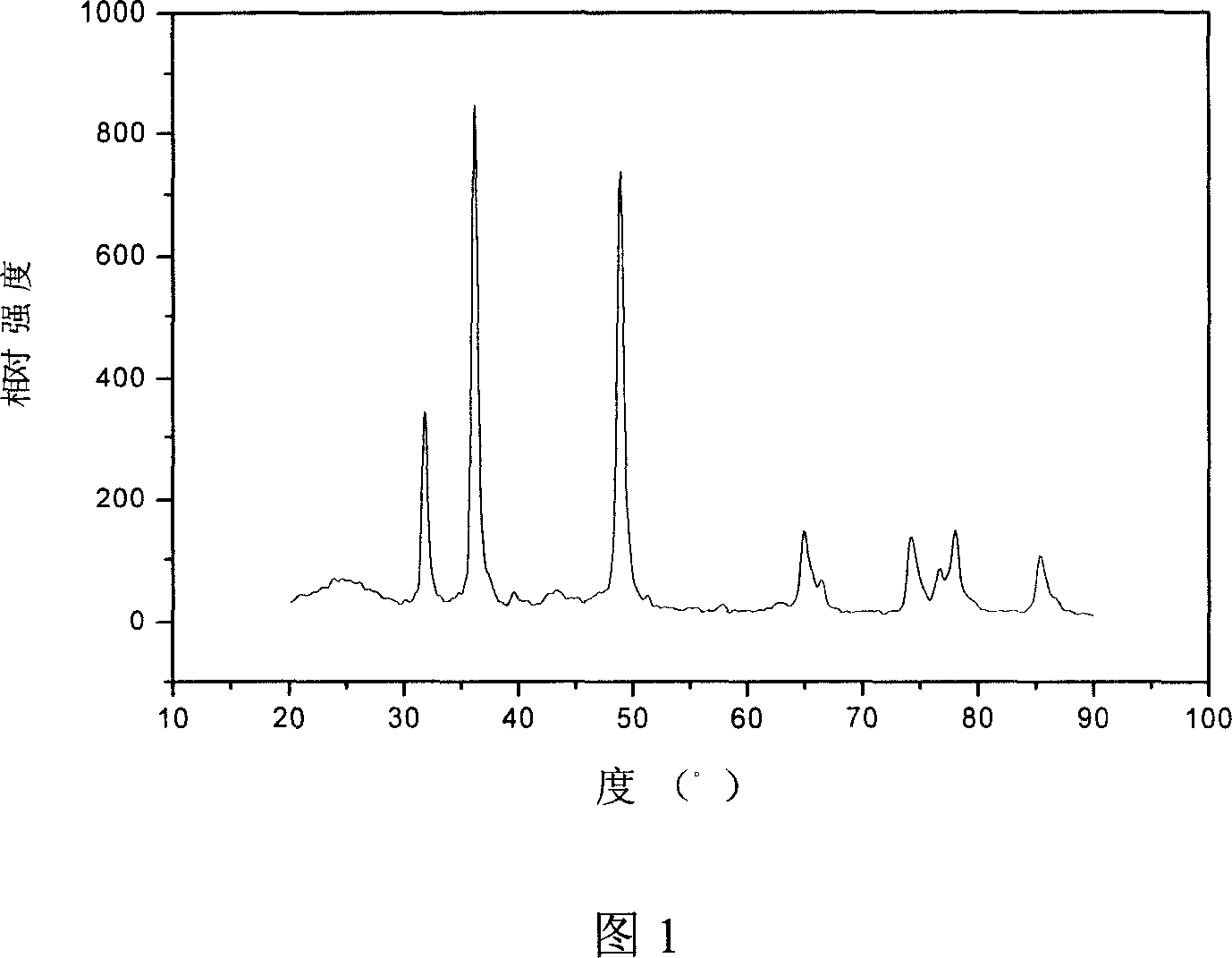
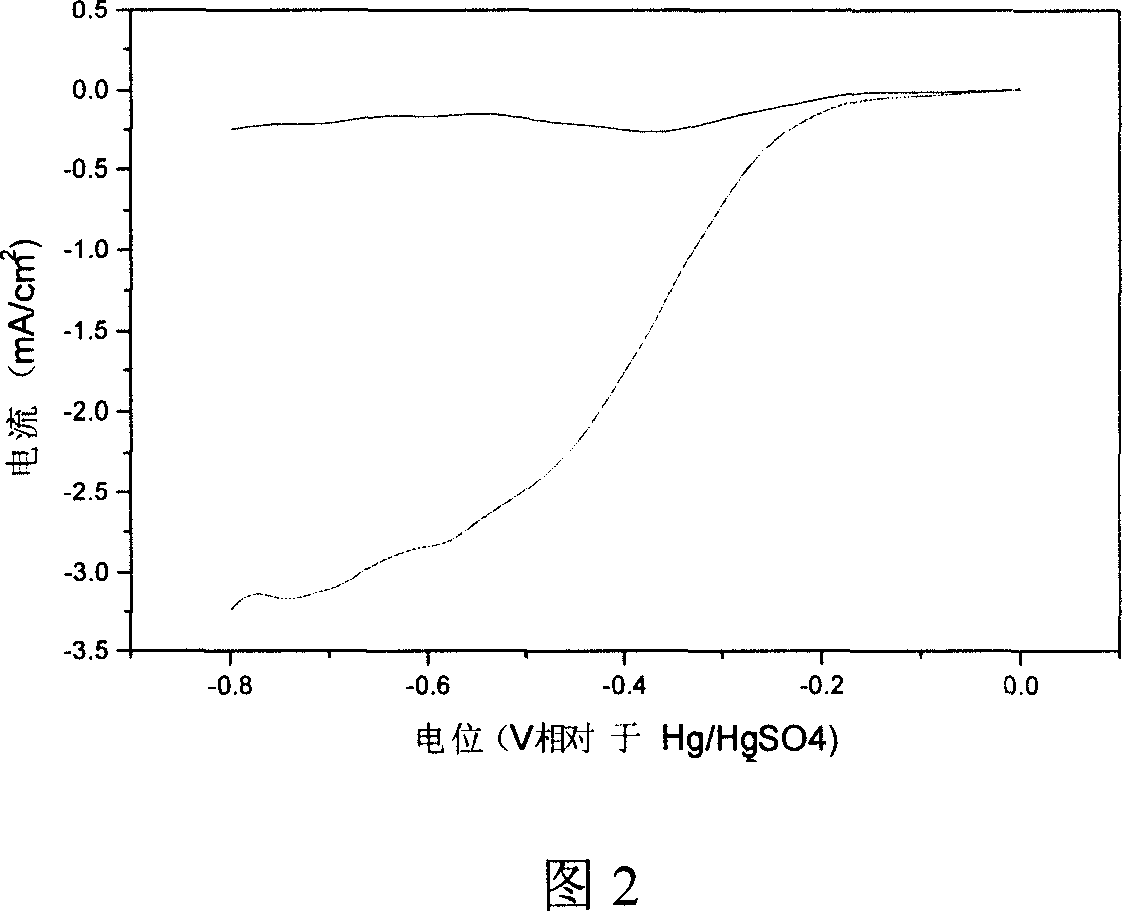
[0022] Molybdenum tetraphenylporphyrin and Vulcan XC-72 activated carbon powder were dissolved in tetrahydrofuran at a mass ratio of 1:9, and ultrasonicated for 60 minutes; the above solution was stirred at room temperature, evaporated to dryness, and dried at 80°C. The obtained powder was sealed in a quartz glass tube, and ammonia gas was introduced at a flow rate of 1 l / min. The heat treatment temperature was controlled at 700°C, and the reaction was carried out at this temperature for 3 hours. Then, the heating was stopped and cooled to room temperature to obtain a MoN / C catalyst. The lens photo shows that the particle size of MoN is 5-6nm. According to the cathode polarization curve attached in Figure 2, the reduction onset potential of oxygen on the catalyst is -0.1V, and the area specific activity is 3.1mA / cm 2 , and the area specific activity of the carbon-supported platinum catalyst is 1.5mA / cm when the relative rotation speed and scan rate are consistent 2 , increased...
Embodiment 2
[0024] Molybdenum octaethylporphyrin and Vulcan XC-72 activated carbon powder were dissolved in acetone at a mass ratio of 1:1, and ultrasonicated for 50 minutes; the above solution was stirred at room temperature, evaporated to dryness, and dried at 60°C. The obtained powder was sealed in a quartz glass tube, and ammonia gas was introduced at a flow rate of 0.5 l / min. The heat treatment temperature was controlled at 700° C., and the reaction was carried out at this temperature for 4 hours. Then, the heating was stopped and cooled to room temperature to obtain a MoN catalyst. The lens photo of accompanying drawing 2 shows that the particle size of the catalyst is 5-6nm. The electrochemical cathodic polarization curve and the chronocurrent curve show that the performance of the catalyst is better than that of the carbon-supported platinum electrocatalyst.
Embodiment 3
[0026] Molybdenum tetramethoxyporphyrin and Vulcan XC-72 activated carbon powder were dissolved in N-N dimethylacetamide at a mass ratio of 2:3, ultrasonicated for 50 minutes; the above solution was stirred at room temperature, evaporated to dryness, and dried at 70°C. The obtained powder was sealed in a quartz glass tube, and ammonia gas was introduced at a flow rate of 2 l / min. The heat treatment temperature was controlled at 600° C., and the reaction was carried out at this temperature for 5 hours. Then, the heating was stopped and cooled to room temperature to obtain a MoN catalyst. The lens photos show that the particle size of the catalyst is 5-6nm. The electrochemical cathodic polarization curve and the chronocurrent curve show that the performance of the catalyst is better than that of the carbon-supported platinum electrocatalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com