New scheme of combining cell factor fusion protein (IL 2/FC) and compound Chinese medicine 861 for enhancing immune response of HB vaccine and breaking immune tolerance of HBV
A technology of cytokines and fusion proteins, applied in the direction of viruses, antiviral agents, fusion polypeptides, etc., can solve the problems that are difficult to remove, cannot achieve virus removal, and the removal rate is less than 30%.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1. Interleukin 2 immunoglobulin Fc (also known as IL-2 / Fc fusion protein) expression Body construction and protein expression
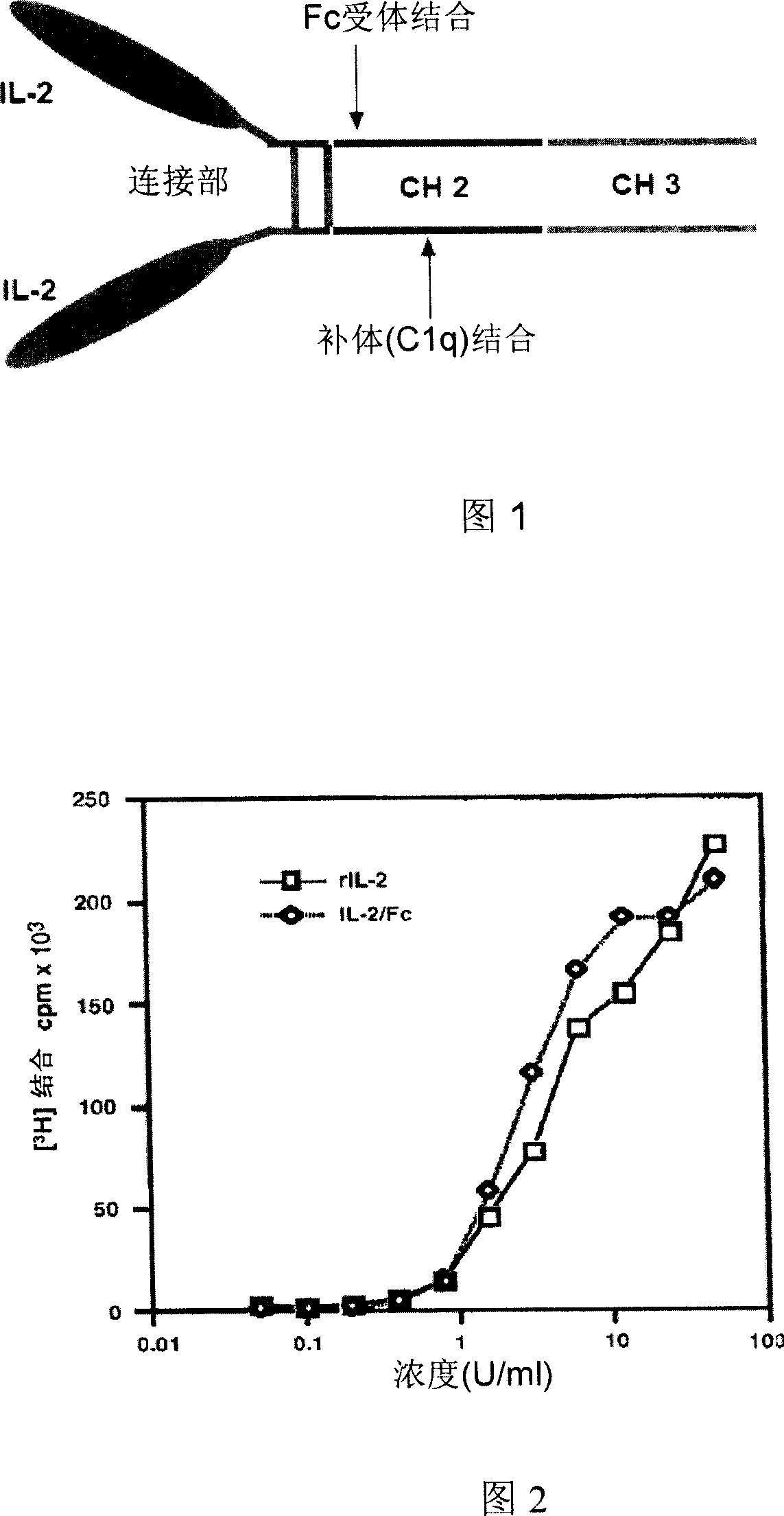
[0048] In order to maintain the full biological activity of interleukin 2 and increase its circulating half-life in vivo. We used genetic engineering to fuse interleukin 2 (IL-2) with the Fc gene of immunoglobulin and express it in mammalian cells, thus creating a new type of IL-2 / Fc fusion protein (Figure 1 , structure of IL-2 / Fc fusion protein).
[0049] Build process:
[0050] Using synthetic primers, mouse IL-2 cDNA (GenBank accessionnumber, NM008366) was cloned from the ATCC 37553 plasmid template. N-terminal primer, plus restriction enzyme site NotI. C-terminal primer with restriction site BamHI appended. Mouse Fcr2a cDNA (GenBank accession number, BC108375) was reverse-transcribed from mRNA of MIgG2a-secreting hybridoma cells (ATCC HB129). The Fcr2a fragment was cloned from the Fcr2a cDNA template by adding a restriction...
Embodiment 2
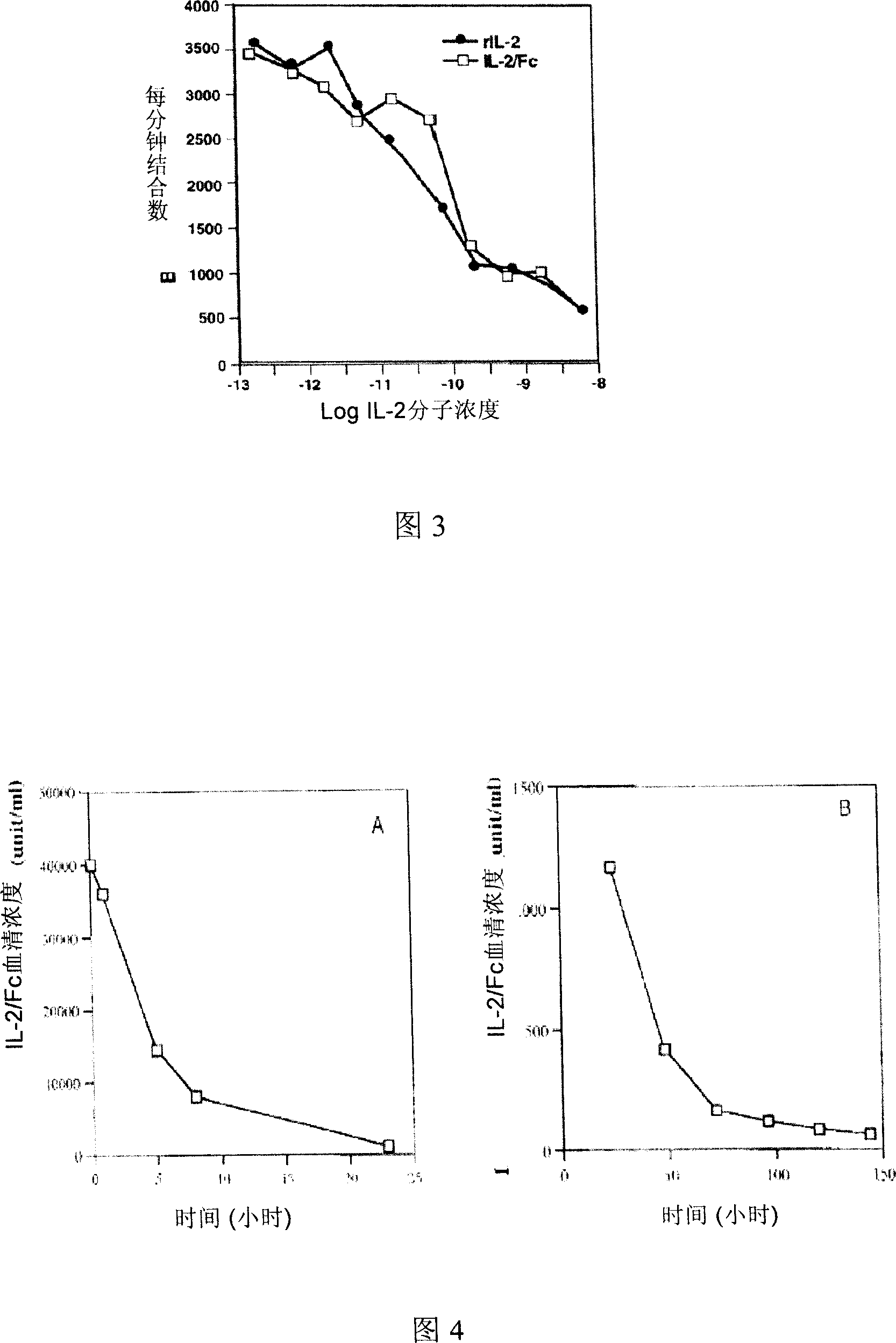
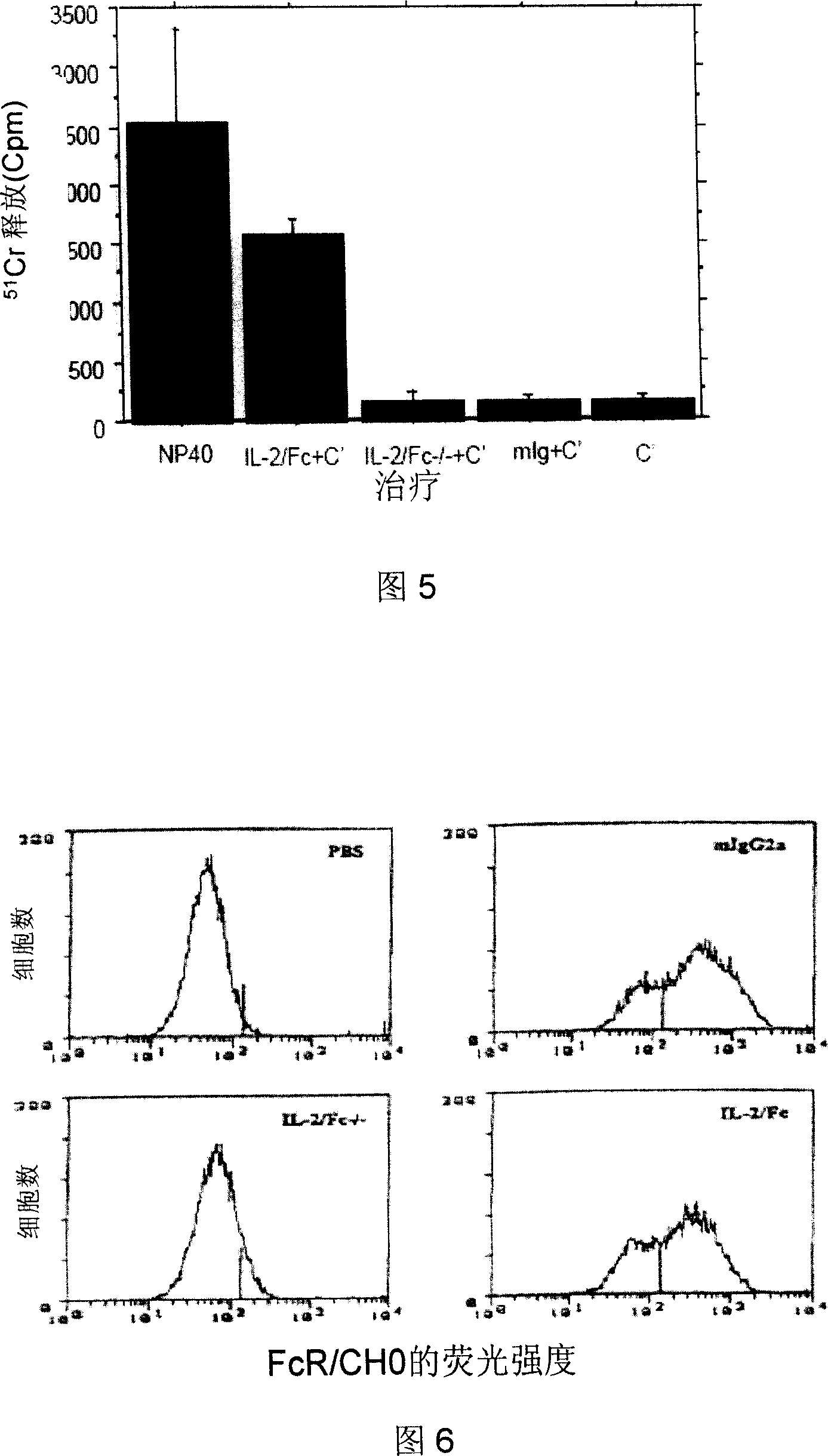
[0057] Example 2.IL-2 / Fc significantly enhances the humoral immune response and enhances the hepatitis B vaccine CD8 against HBV + T cell cellular immunity
[0058] Since IL-2 / Fc has all the biological activities of IL-2 and has a circulating half-life of up to 20 hours, we use IL-2 / Fc as an immunopotentiator to enhance humoral and cellular immune responses susceptible to immune challenge. We first adopted the protocol of intraperitoneal injection of IL-2 / Fc fusion protein. Balb / c mice (commercially purchased from Beijing Weitong Lihua Animal Co., Ltd., certificate number 0060937) received intradermal hepatitis B vaccine immunization (recombinant yeast hepatitis B vaccine, a product of Beijing Tiantan Biotechnology Co., Ltd., batch number 20080304, each Rats were subcutaneously injected with 1 μg on each side of the groin, the total dose was 2 μg / rat), and from the next day, they received a daily intraperitoneal injection of IL-2 / Fc (commercially purchased from Chimerigen C...
Embodiment 3
[0062] Embodiment 3. Chinese medicine compound Danqi mixture (new prescription) (hereinafter referred to as compound recipe 861 (new prescription))
[0063] See references 1 and 2 for the old prescription of Chinese medicine 861. The present invention relates to an improved Fu Fang 861 (Xin Fang), for details, please refer to Reference 3.
[0064] 1. Containing ingredients: Danshen, Astragalus, Cordyceps mycelium, Bupleurum, Radix Paeoniae Rubra, Angelica, Safflower, Rhizoma Cyperi, Tangerine Peel, Millet Spatholobus, Chuanxiong and other eleven herbs.
[0065] 2. Production process: firstly, the crude drugs of each single herb are extracted, concentrated, dried, and granulated to make granules. Then each granule (prepared and provided by Tianjiang Pharmaceutical Factory in Jiangyin City, Jiangsu Province or Beijing Capital Dadi Pharmaceutical Co., Ltd., providing dosage form as granule) is uniformly mixed with the following doses in parts by weight to form Compound 861 (ne...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com