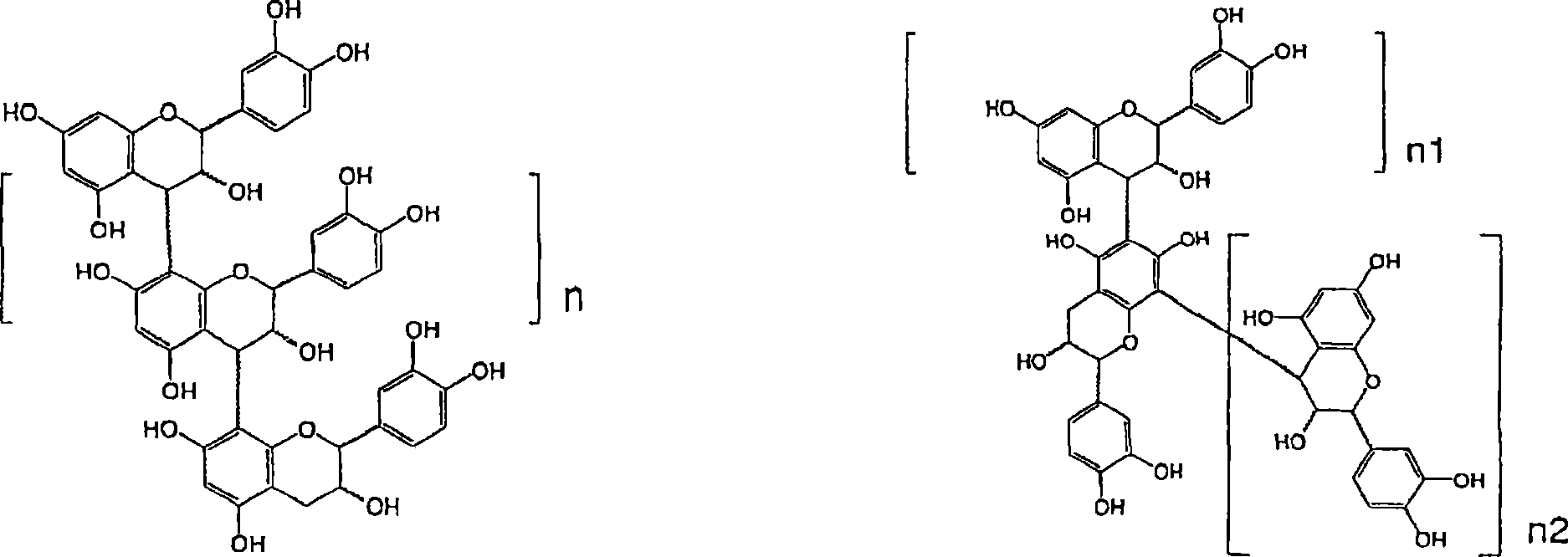
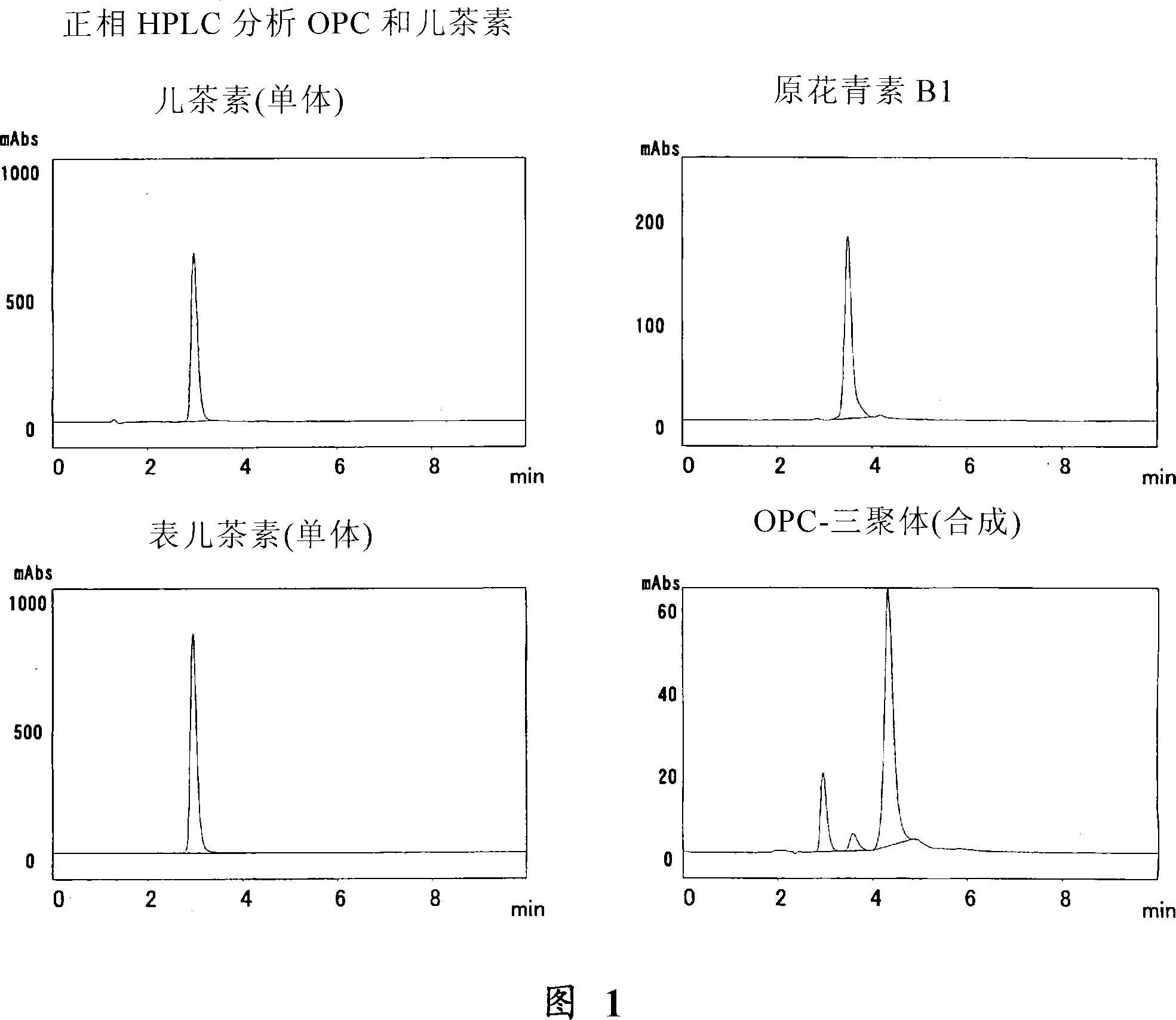Method for analyzing oligomeric proanthocyanidin (opc)
A technology of oligomeric proanthocyanidins and anthocyanins, which is applied in the field of oligomeric proanthocyanidins, can solve the problem of no qualitative and quantitative determination of OPC, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The research of embodiment 1 acid hydrolysis condition
[0060] Observe the change over time when the pine bark extract (flavangenol) is decomposed by acid. Pine bark extract (1 mg) was dissolved in 1 ml of 0.6N HCl / BuOH, and the solution was heated in a hot water bath at 90°C. Sampling was performed every 20 minutes from 20 minutes to 140 minutes. The sample was diluted 1:10 with butanol, and the visible absorption spectrum at 400-700 nm was measured. Absorption maximum occurs at 551 nm for all samples. The measurement results are shown in Table 1.
[0061] Table 1 Changes of acid decomposition of pine bark extract over time
[0062] time
[0063] The result was a slow increase in absorbance over time during acid decomposition, reaching a peak at 120 min. Based on this result, in subsequent tests, the hydrolysis period was set at 120 minutes (2 hours).
Embodiment 2
[0064] Production and determination of anthocyanins in embodiment 2 acid hydrolysis
[0065] In a glass test tube, a sample (0.5 mg) containing proanthocyanidins was dissolved in 1 ml of 0.6N HCl / butanol, and the resulting solution was left to stand in a water bath at 90° C. for 2 hours. After the reaction was completed, the absorption spectrum at 700-400 nm was measured with UV-265 (Shimadzu Corp.), and the absorbance at 551 nm was measured. Under the following conditions, use HPLC to analyze the solution after the reaction to determine anthocyanins:
[0066] Column: YMC-ODS-A312, 6mm×150mm
[0067] Mobile phase: CH 3 COOH:MeOH:H 2 O=15:17.5:67.5
[0068] Detection: A520nm (measured at 400-600nm using PDA)
[0069] The standard substances delphinidine, cyanidin and geranium pigment used for analysis were purchased from Funakoshi. The standard substance delphinium is set to flow out in 4.2 minutes, and its λ max is 535nm; cyanidine flows out in 5.5 minutes, its λ max ...
Embodiment 3
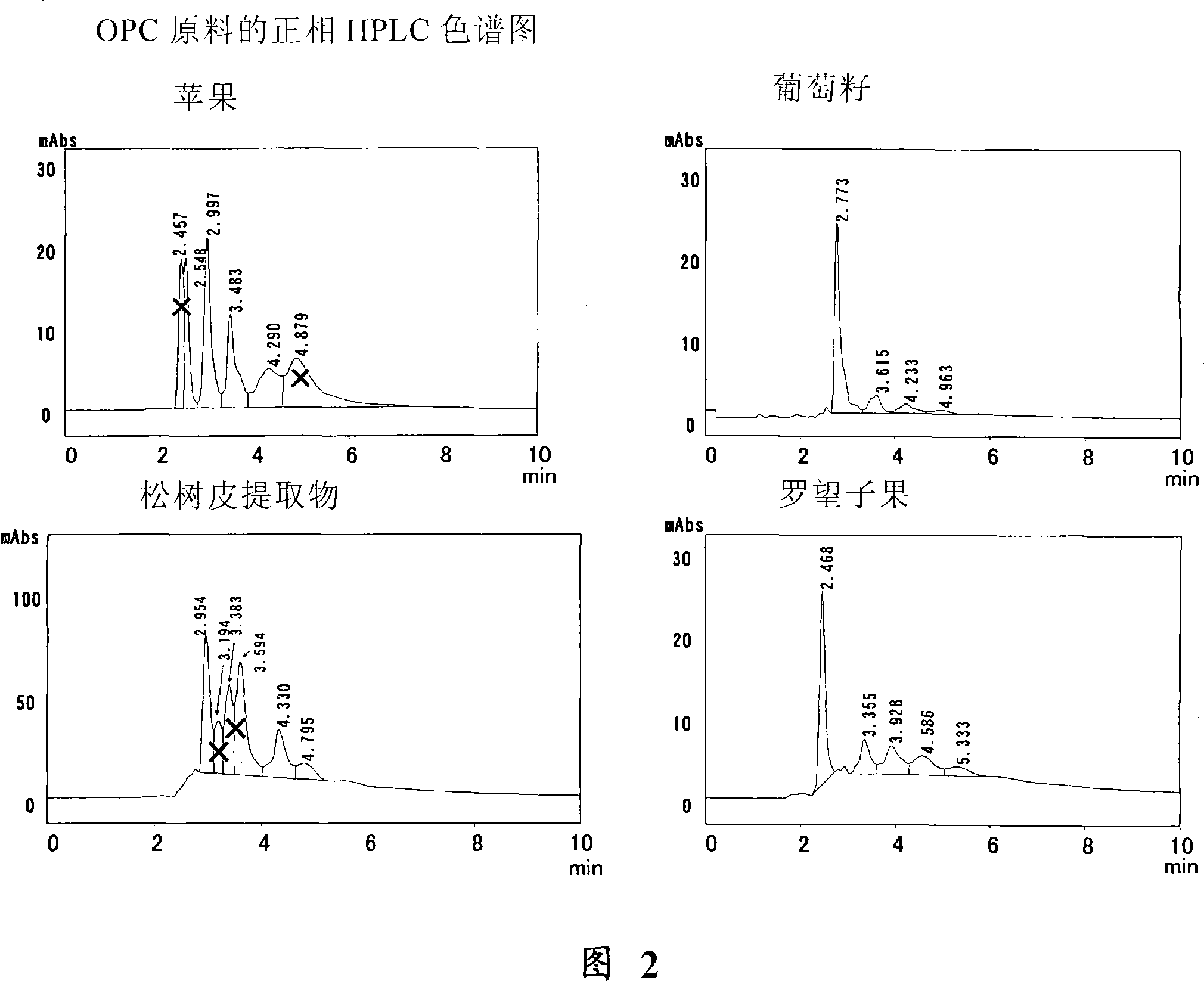
[0077] Example 3 Analysis of catechins, dimers and trimers by normal phase HPLC
[0078] Standard materials for catechins and proanthocyanidins were analyzed using normal phase HPLC under the following conditions:
[0079] The samples (0.1 mg of each sample) of (+)-catechin (Nacalai Tesque), (-)-epicatechin (Wako Pure Chemical Industries), proanthocyanidin B1 (Funakoshi) were respectively dissolved in 1 ml mobile phase, and used The above solution was filtered with a 0.45 μm filter, and then subjected to HPLC analysis under the following conditions.
[0080] The trimer was synthesized using the method described in Example 5 below.
[0081] Column: Inertsil SIL, 4.6mm×150mm
[0082] Mobile phase: hexane: MeOH: THF: HCOOH=45:40:14:1
[0083] Detection: A280nm (measured at 240-400nm using PDA)
[0084] Under these conditions, monomers ((+)-catechin and (-)-epicatechin) elute at 2.9 minutes, dimers at 3.6 minutes and trimers at 4.3 minutes.
[0085] The chromatograms of thes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com