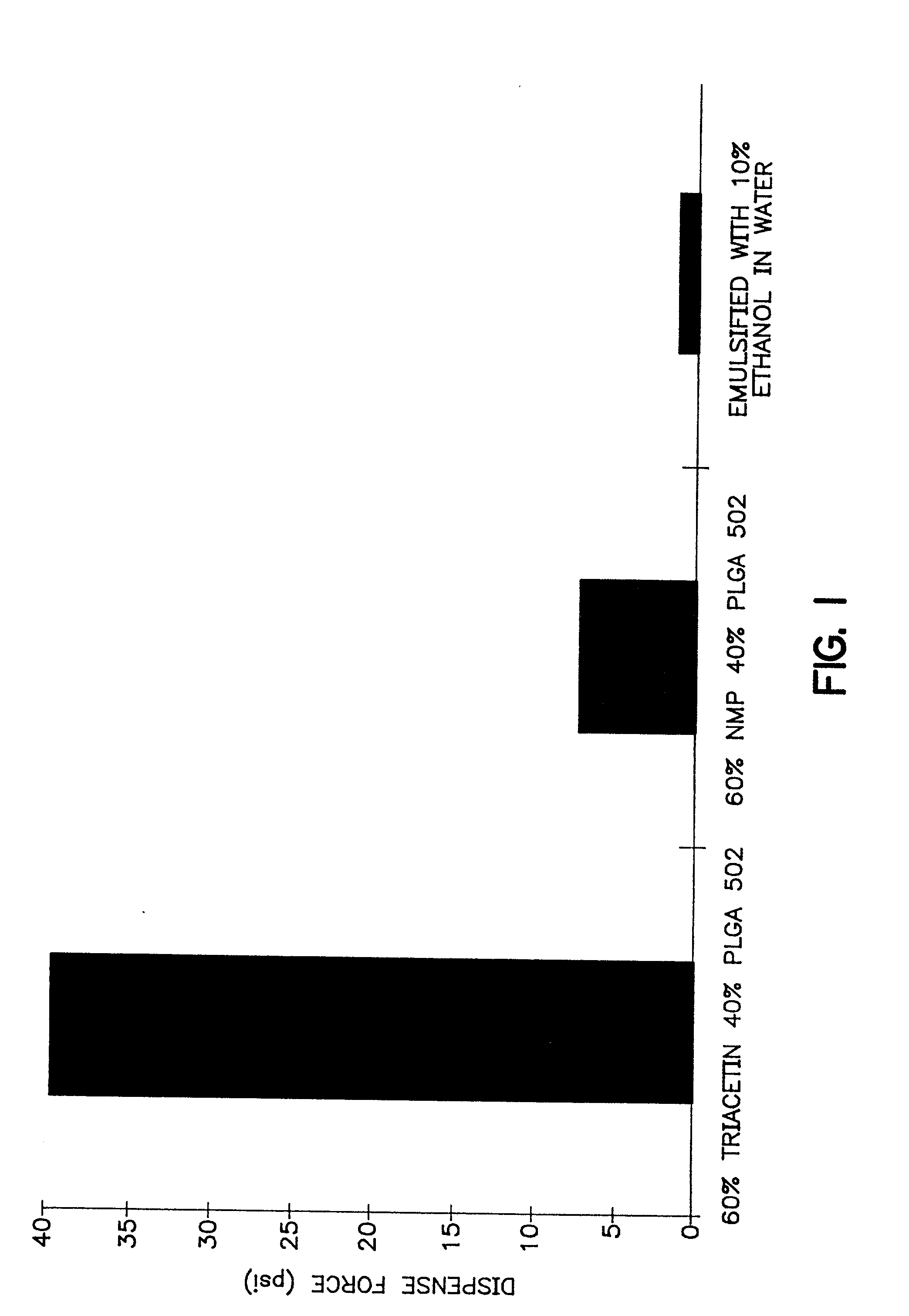
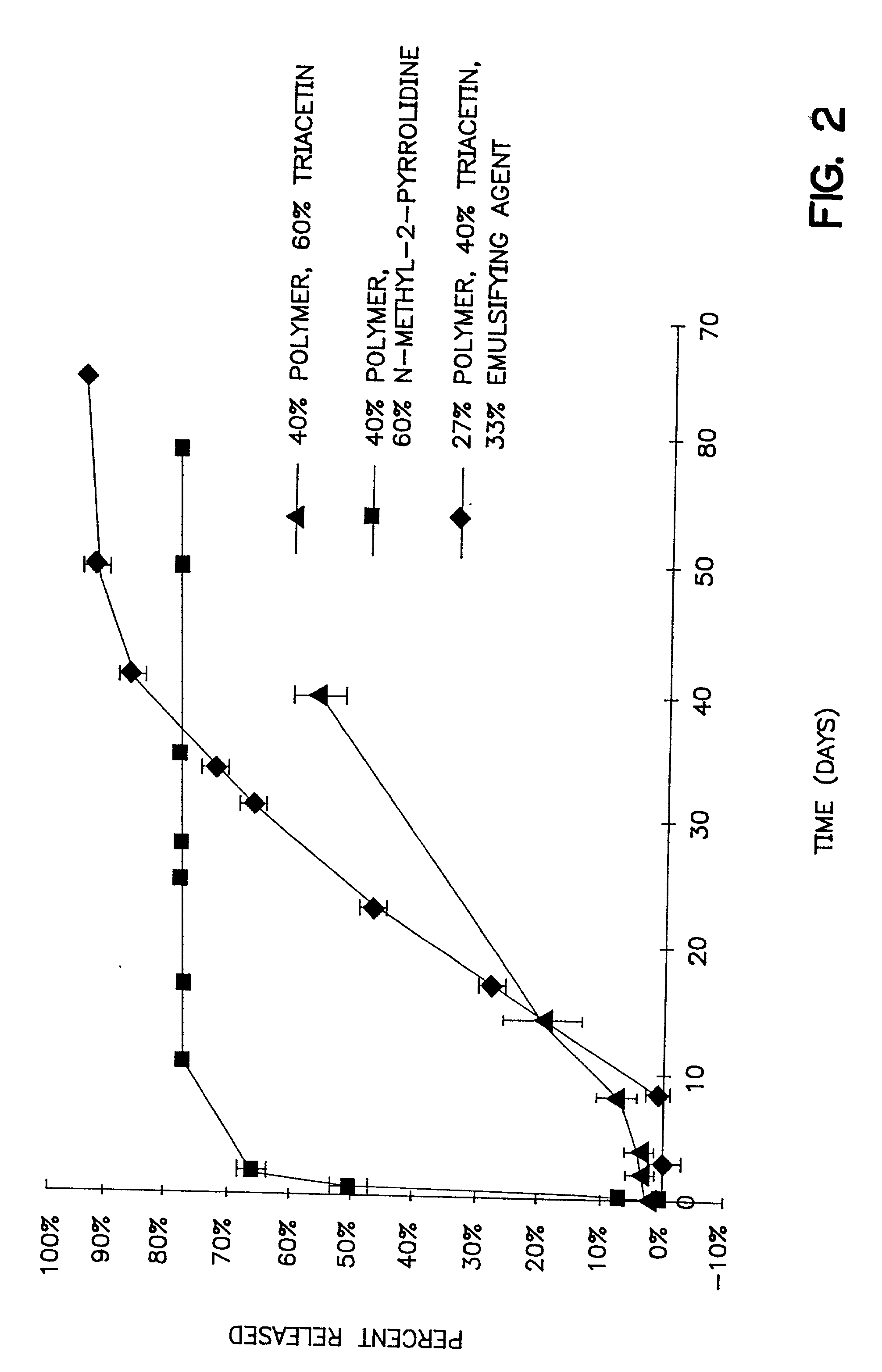
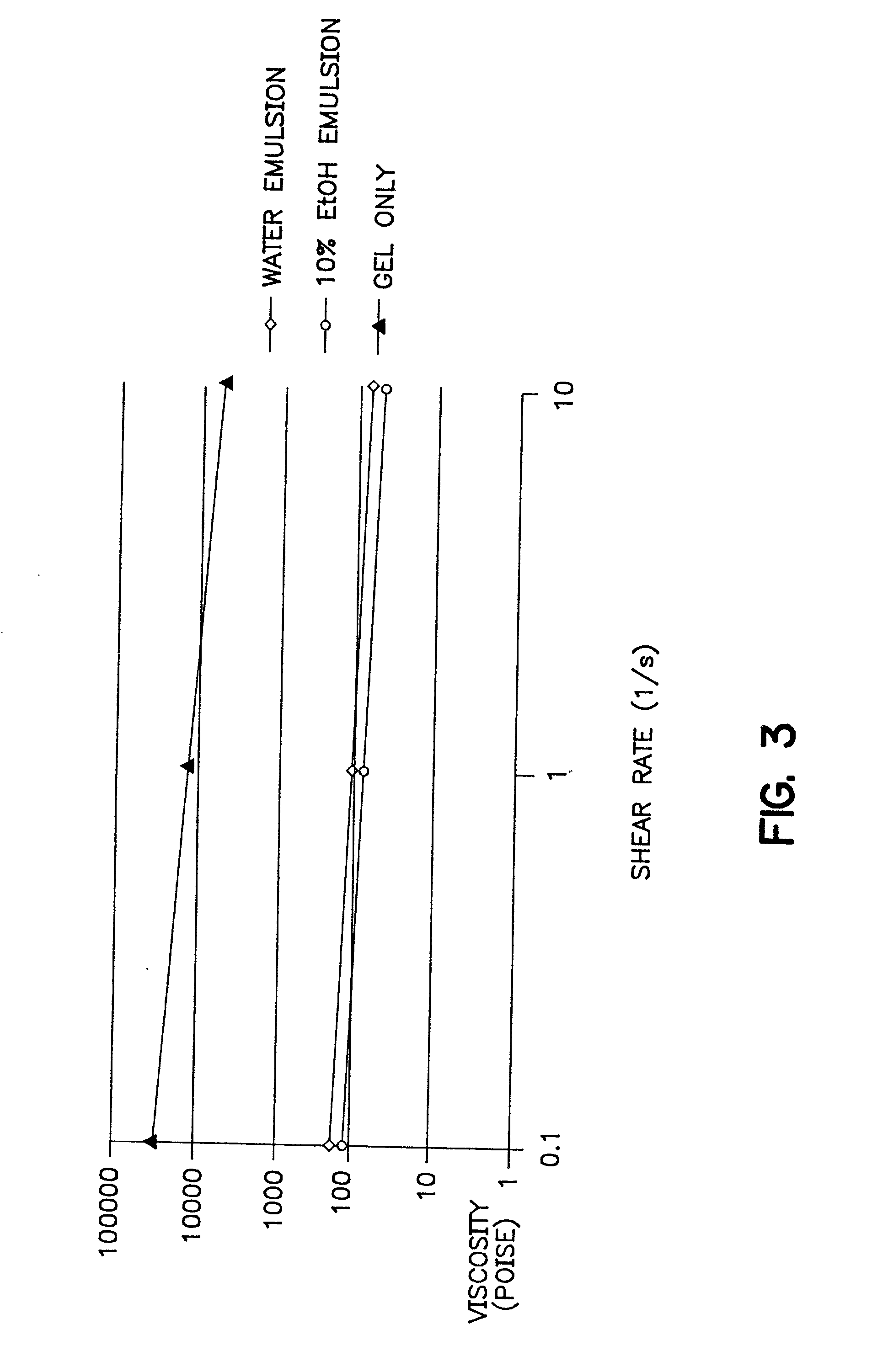
Injectable depot gel composition and method of preparing the composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
[0063] Lysozyme particles were made by spray drying 50% sucrose and 50% chicken lysozyme (on a dry weight basis).
[0064] A viscous gel material was prepared by heating 60% by weight of triacetin with 40% by weight of a 50:50 lactic acid:glycolic acid copolymer to 37.degree. C. overnight. The viscous gel was allowed to cool to room temperature while mixing continued. The lysozyme particles were added to the viscous gel in a ratio of 20:80 lysozyme particles:gel (by weight). The combination was mixed for 5 minutes. Immediately prior to use, a 10% ethanol, 90% isotonic saline solution was added as the emulsifying agent. The emulsifying agent comprised 1 / 3 of the total injectable depot gel composition. 0.5 grams of this injectable depot composition was then injected into a rat.
[0065] In accordance with various aspects of the present invention, one or more significant advantages can be obtained. More specifically, using simple processing steps, one can obtain a depot gel composition that ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com