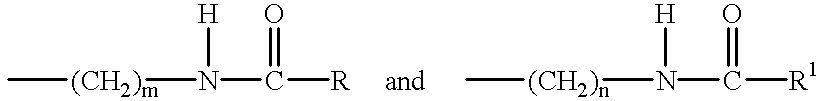Antiperspirant compositions
a technology of compositions and antiperspirants, applied in the field of antiperspirant compositions, can solve the problems of white marks, visible white deposits, formulations containing gellants, etc., and achieve the effect of superior properties and low visible residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
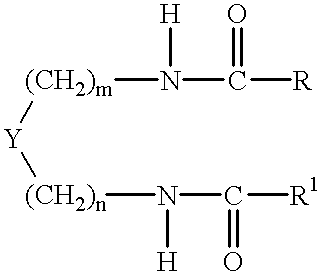
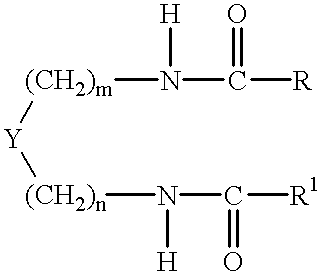
Preparation of 1,2-diamidocyclohexane Compounds
[0140] The following general procedure was employed, based on that described by K Hanabusa, M Yamada, M Kimura and H Shirai, in Prominent Gelation and Chiral Aggregation of Alkylamides Derived from Trans-1,2-Diaminocyclohexane, Angew. Chem. Int.Ed. Engl., 35 (17), 1949 (1996).
[0141] The reaction vessel was a three necked one liter round bottom flask equipped with overhead mechanical stirrer, water condenser and 250 ml pressure equalising dropping funnel. 1,2-diaminocyclohexane in 300 mls of toluene was placed in the flask followed by triethylamine. The acid chloride in 150 mls of toluene was placed in the pressure equalising funnel. The solution of the amine was taken to reflux after which the acid chloride was added dropwise. A white dense precipitate (triethylamine hydrochloride) forms almost immediately. After 4 hours of reflux the triethylamine hydrochloride is filtered off whilst the reaction mixture is still hot and the toluene is...
example 2
[0196] A number of suspension stick compositions, as set out in the tables below were prepared. The materials used, and their proprietary names, were as follows:
[0197] 1) Volatile cyclic silicone (cyclomethicone) DC 245 (Dow Corning)
[0198] 2) Polypropyleneglycol 14 butylether (Fluid AP from Union Carbide)
[0199] 3) Polydecene (Silkflo 364 NF from Albemarle)
[0200] 4) Isostearyl alcohol (abbreviated to ISA--Prisorine 3515 from Unichema)
[0201] 5) C12-15 alkyl benzoate (Finsolv TN from Finetex)
[0202] 6) Al / Zr Tetrachlorohydrex glycine complex (AZAG -7167 from Summit).
[0203] 7) 12-hydroxystearic acid (Caschem)
[0204] The method of preparation was as follows:
[0205] The liquids and the structurant(s) were first mixed together in a flask and heated to just above the dissolution temperature of the structurant(s). Gentle stirring was provided with a Hydolph mixer. Once all the structurant was dissolved the antiperspirant active was added and the whole mixture cooled to 5-10.degree. C. above the...
example 3
Emulsion Sticks
[0209] A number of emulsion stick compositions were prepared. Some of the materials used were listed at the beginning of Example 2. Others were
[0210] 8) Cetyl dimethicone copolyol (Abil EM90 emulsifier from Th. Goldschmidt)
[0211] 9) 50% aqueous solution of Al / Zr pentachlorohydrate (Zirkonal 50 from Giulini)
[0212] The liquids and emulsifier were mixed together in a beaker and heated to 5-10.degree. C. above the dissolution temperature of the amido-containing structurant, while gently stirring with a Silverson head. The structurant was added. Next the antiperspirant active phase, preheated to the same temperature, was added slowly. During this addition, the stirring was increased to 8000 rpm. Once the addition was complete the mixture was stirred for a further 5 minutes at 8000 rpm without any cooling. The process was then stopped and the mixture transferred into stick barrels and left at room temperature to cool.
4 Formulation No: 3.1 3.2 3.3 3.4 3.5 3.6 3.7 Parts by we...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com