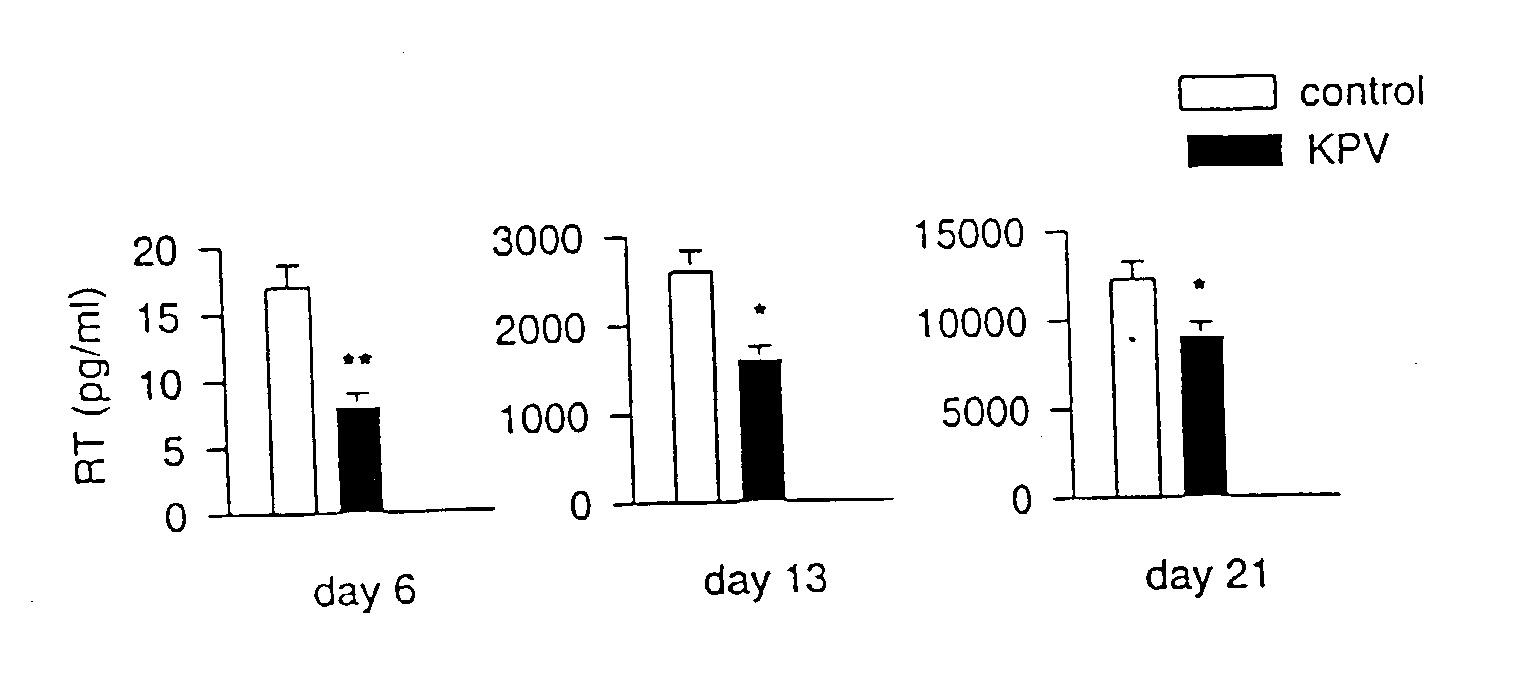
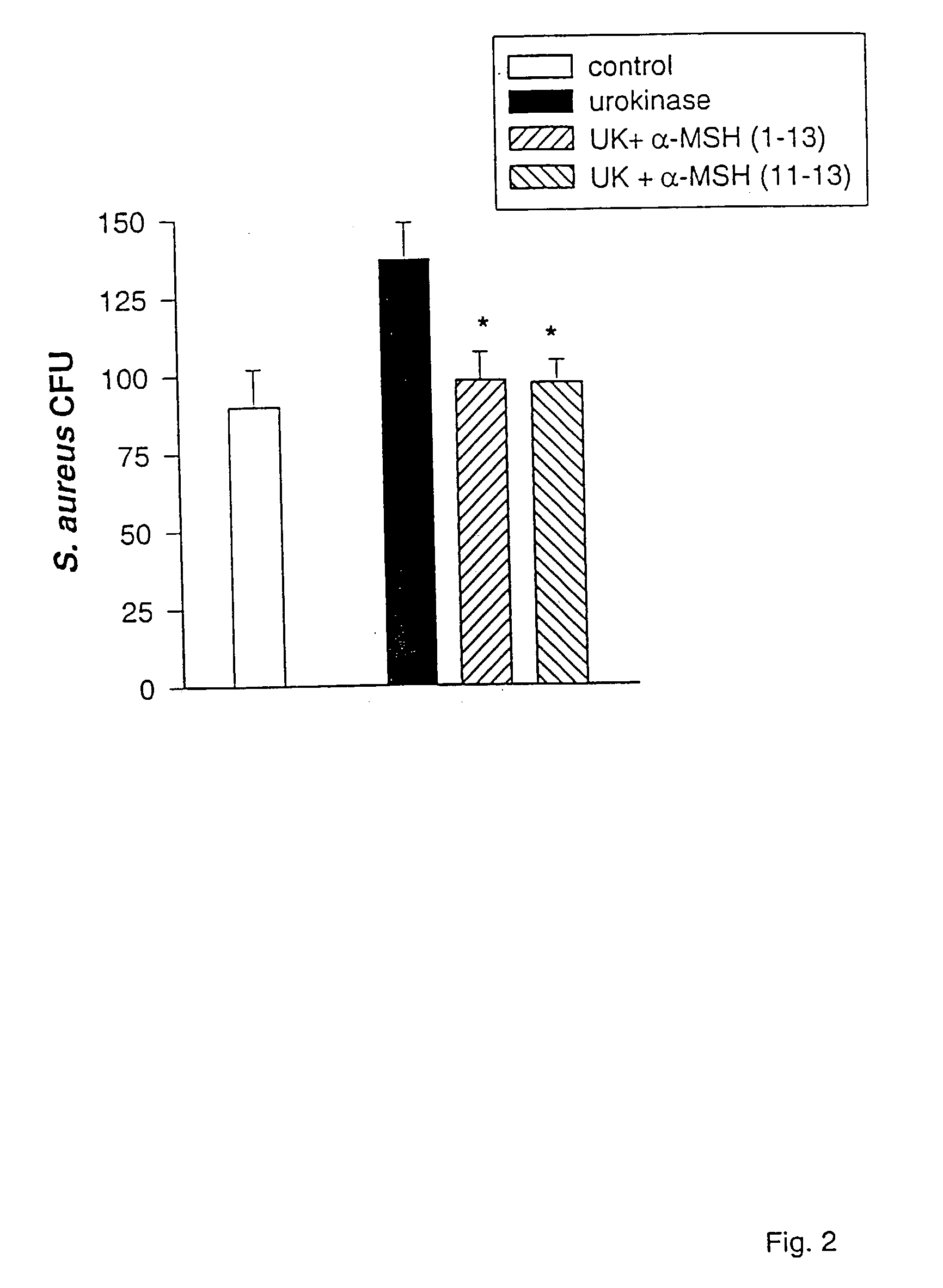
Lys-pro-val dimer, formulations and applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example ii
[0043] This example illustrates the anti-fungal properties of .alpha.-MSH and / or its derivatives against Candida albicans.
[0044] Clinical isolates of C. albicans were also obtained from the collection of the Department of Microbiology, Ospedale Maggiore di Milano. Cultures of C. albicans were maintained on Sabouraud's agar slants and periodically transferred to Sabouraud's agar plates and incubated for 48 hours at 28.degree. C. To prepare stationary growth-phase yeast, a colony was taken from the agar plate, transferred into 30 ml of Sabouraud-dextrose broth, and incubated for 72 hours at 32.degree. C. Cells were centrifuged at 1000.times.g for ten minutes, and the pellet was washed twice with distilled water. Cells were counted and suspended in Hank's balanced salt solution ("HBSS") to the desired concentration. Viability, determined by exclusion of 0.01% methylene blue, remained greater than 98%.
[0045] At 1.times.10.sup.6 / ml in HBSS, these fungi were incubated in the presence or a...
example iii
[0047] This example compares the anti-infection activities of .alpha.-MSH and / or its derivatives to fluconazole, an established anti-fungal agent.
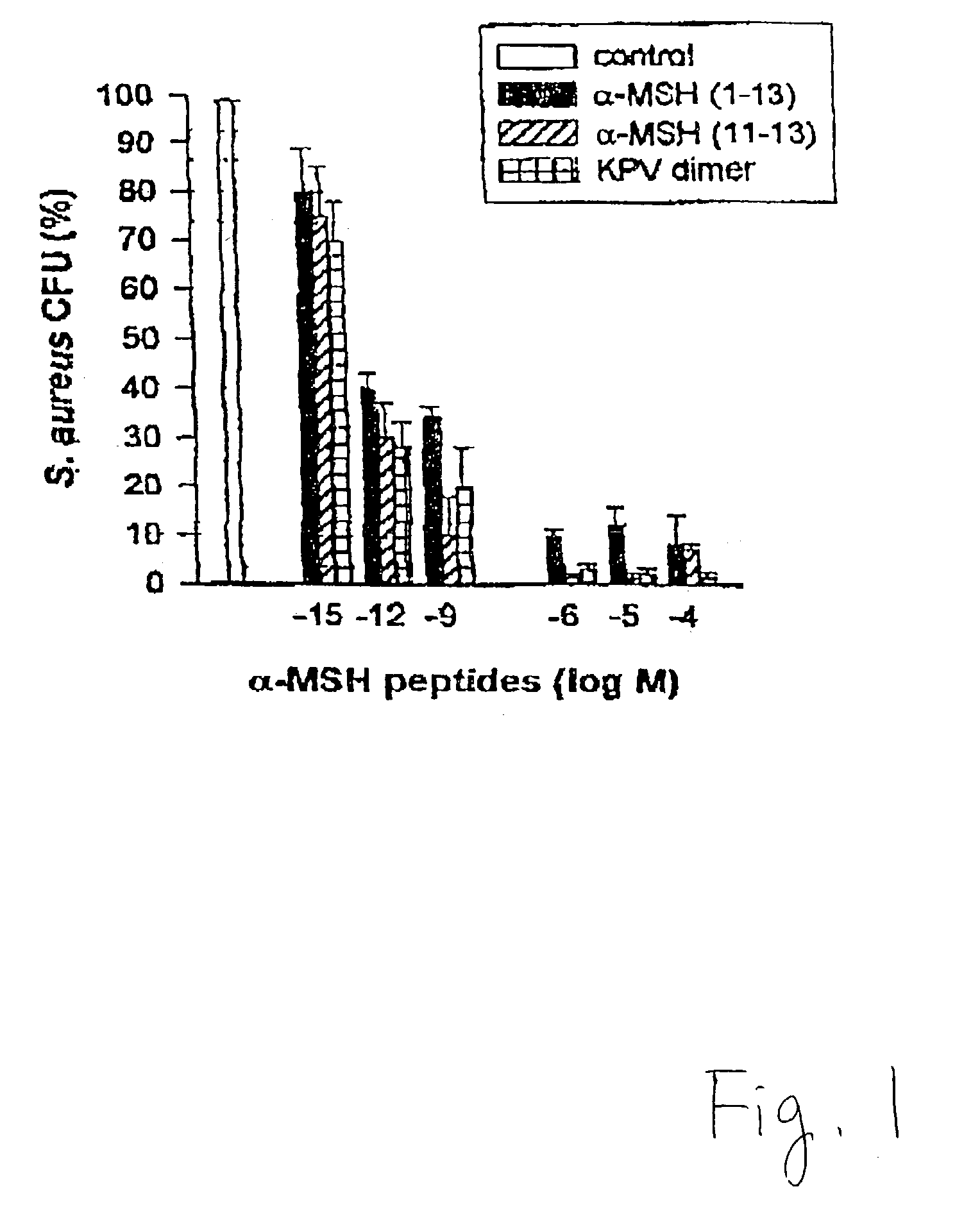
[0048] .alpha.-MSH (1-13) (SEQ. ID. NO.4), (4-10) (SEQ. ID. NO.2), (6-13) (SEQ. ID. NO.3), (11-13) (SEQ. ID. NO. 1), ACTH (1-39), (18-39), and fluconazole, at concentrations of 10.sup.-6 to 10.sup.-4 M, were tested against C. albicans using the same procedures as in Example II. FIG. 4 shows that compared with fluconazole, .alpha.-MSH (11-13) (SEQ. ID. NO. 1), (6-13) (SEQ. ID. NO. 3), and (1-13) (SEQ. ID. NO. 4) were most effective against C. albicans. Their inhibitory activities were similar to fluconazole at the same molar concentration. In contrast, the "core" .alpha.-MSH sequence (4-10) (SEQ. ID. NO. 2), which has behavioral effects but little anti-inflammatory activity, caused approximately 50% inhibition of colony forming units (CFU). Although this inhibitory effect was substantial (p<0.01 vs. control), it was significantly less poten...
example iv
[0050] This example illustrates that .alpha.-MSH and its derivatives inhibit the germination or germ tube formation of C. albicans. Germ tube formation is a significant part of the pathogenesis of C. albicans infection. This pathogenesis involves adhesion to host epithelial and endothelial cells and morphologic switching from the ellipsoid blastospore to various filamentous forms, e.g. germ tubes, pseudohyphae, and hyphae. Gow, N. A., Germ Tube Growth of Candida albicans, Curr. Topics Med. Myco. 8, 43-55 (1997).
[0051] C. albicans from stationary phase cultures were washed twice with distilled water and suspended in HBSS to a final concentration of 2.times.10.sup.6 / ml. Hyphal growth was induced by addition of 10% inactivated horse serum (GIBCO / BRL, Paisley, Great Britain) to yeast incubated for 45 minutes at 37.degree. C. with continuous shaking. Horse serum was then removed by washing cells twice with HBSS, and incubation was further continued for 60 minutes at 37.degree. C. in the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Area | aaaaa | aaaaa |
| Toxicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com