Synthetic genes for plant gums and other hydroxyproline-rich glycoproteins
a technology of plant gums and glycoproteins, which is applied in the field of synthetic genes for plant gums and other hydroxyproline-rich glycoproteins, can solve the problems of reducing the number of cells receiving, affecting the quality of plant gums, so as to achieve the effect of maximizing the number of cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Determination of the Peptide Sequence of Acacia Gum Arabic Glycoproteins
[0182] In this example, GAGP (SEQ ID NO:15) was isolated and (by using chymotrypsin) the deglycosylated polypeptide backbone was prepared. Although GAGP does not contain the usual chymotryptic cleavage sites, it does contain leucyl and histidyl residues which are occasionally cleaved. Chymotrypsin cleaved sufficient of these "occasionally cleaved" sites to produce a peptide map of closely related peptides.
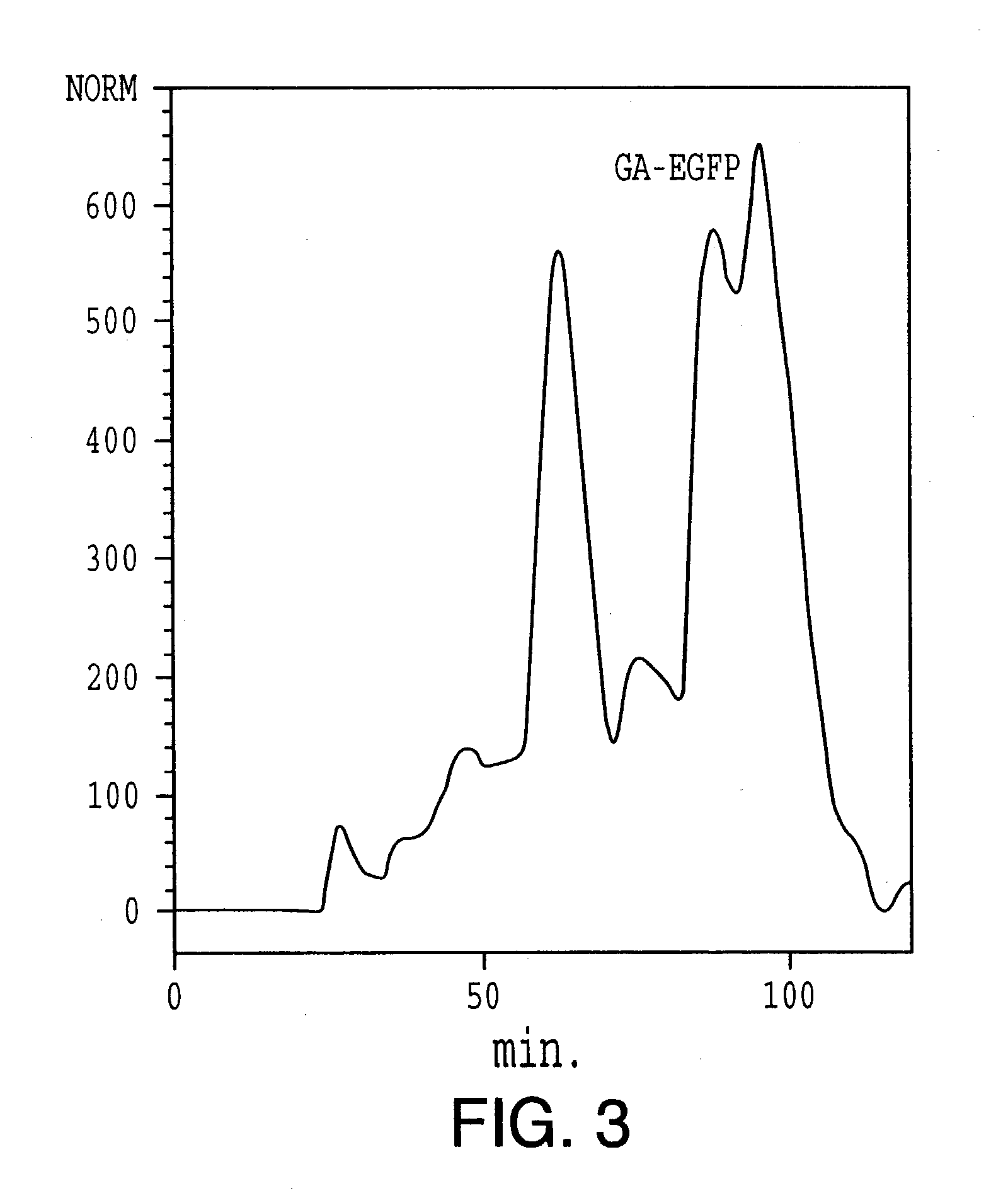
[0183] Purification and Deglycosylation of GAGP (SEQ ID NO:15). GAGP was isolated via preparative Superose-6 gel filtration. Anhydrous hydrogen fluoride deglycosylated it (20 mg powder / mL HF at 4.degree. C., repeating the procedure twice to ensure complete deglycosylation), yielding dGAGP which gave a single symmetrical peak (data not shown) after re-chromatography on Superose-6. Further purification of dGAGP by reverse phase chromatography also gave a single major peak, showing a highly biased but constant ami...
example 2
Construction of Synthetic HRGP Gene Cassettes
[0186] Synthetic gene cassettes encoding contiguous and noncontiguous Hyp modules are constructed using partially overlapping sets consisting of oligonucleotide pairs, "internal repeat pairs" and "external 3'- and 5'-linker pairs" respectively, all with complementary "sticky" ends. The design strategy for the repetitive HRGP modules combines proven approaches described earlier for the production in E. coli of novel repetitive polypeptide polymers (McGrath et al. [1990] Biotechnol. Prog. 6:188), of a repetitious synthetic analog of the bioadhesive precursor protein of the mussel Mytilus edulis, of a repetitive spider silk protein (Lewis et al. [1996] Protein Express. Purif. 7:400), and of a highly repetitive elastin-like polymer in tobacco [Zhang, X., Urry, D. W., and Daniell, H. "Expression of an environmentally friendly synthetic protein-based polymer gene in transgenic tobacco plants," Plant Cell Reports, 16: 174 (1996)].
[0187] The basi...
example 3
Isolation of Tomato P1 Extensin cDNA Clones
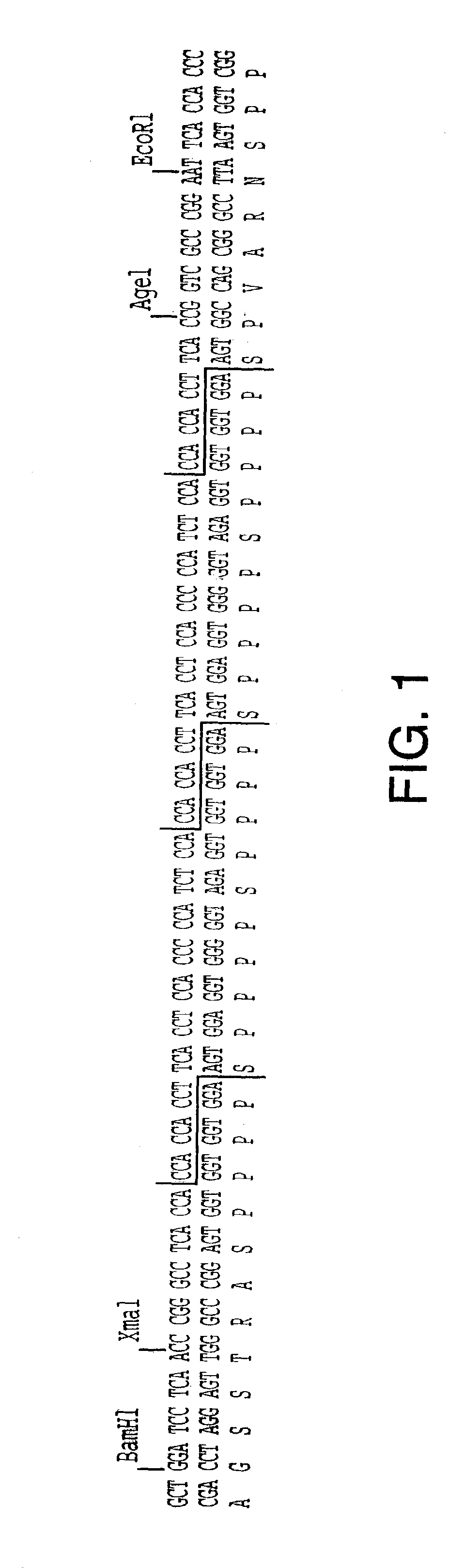
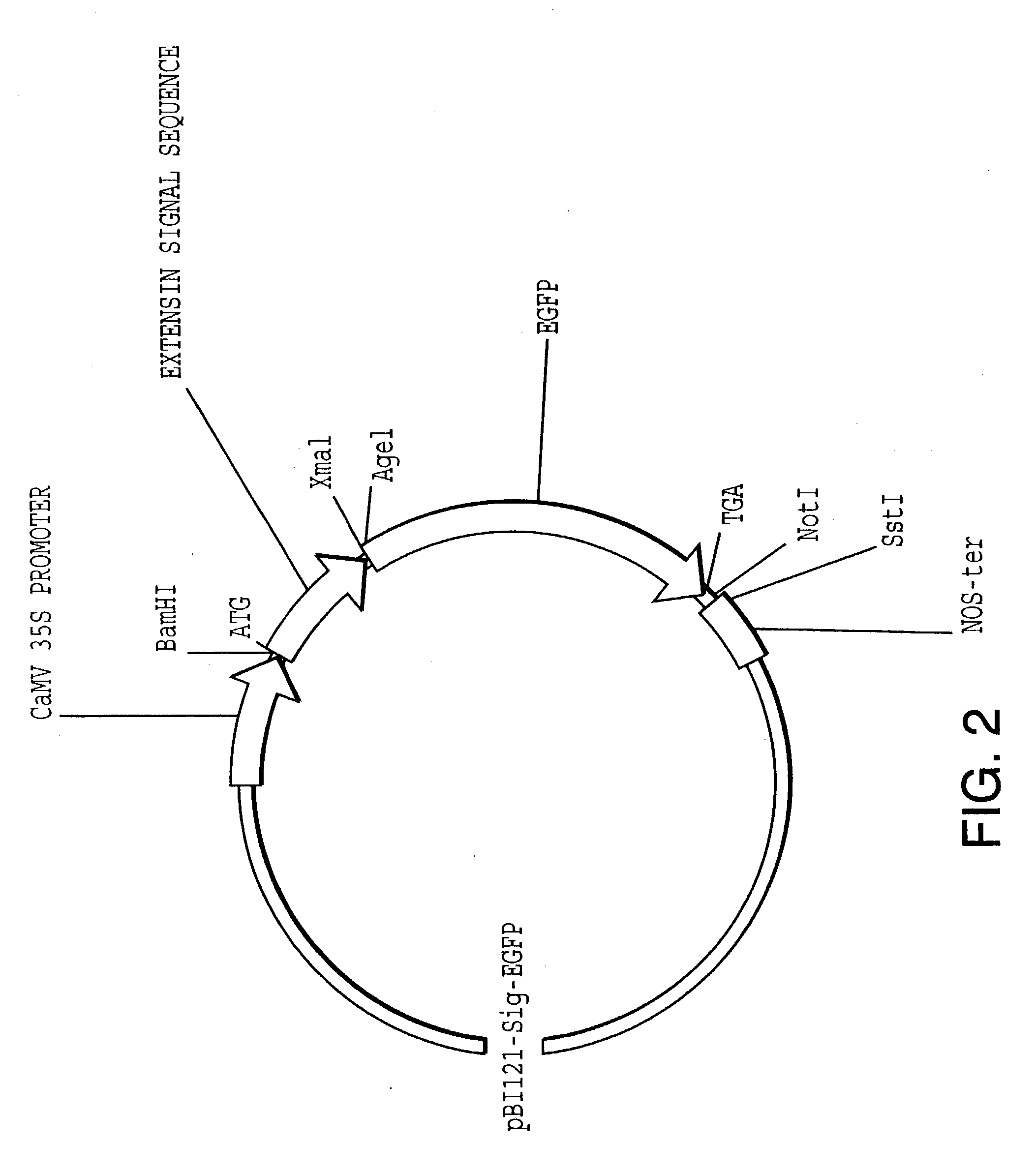
[0211] In order to obtain the tomato P1 extensin signal sequence (i.e., signal peptide), P1 extensin cDNA clones were isolated using oligonucleotides designed after the P1-unique protein sequence (SEQ ID NO:51): Val-Lys-Pro-Tyr-His-Pro-Thr-Hyp-Val-Tyr-Lys. When present at the N-terminus of a protein sequence, the P1 extensin signal sequence directs the nascent peptide chain to the ER.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tm | aaaaa | aaaaa |
| optical density | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com