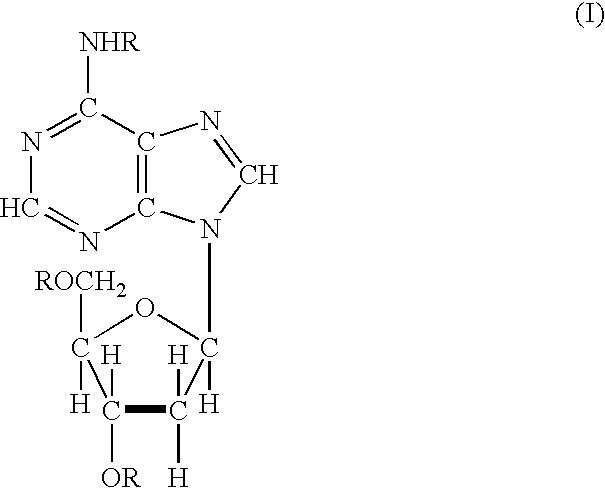
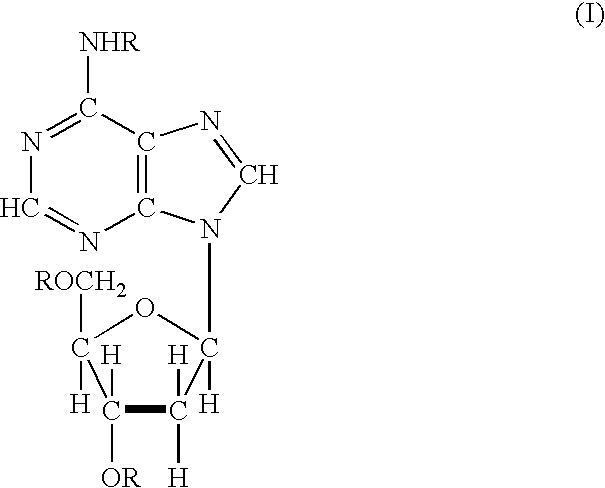
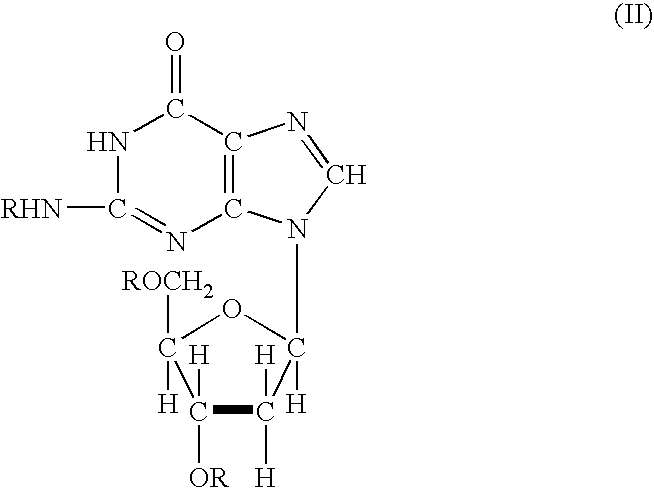
Acyl deoxyribonucleoside derivatives and uses thereof
a technology of acyl deoxyribonucleoside and deoxyribonucleoside, which is applied in the direction of sugar derivatives, biocide, plant growth regulators, etc., can solve the problems that the method of introducing deoxyribonucleoside in sufficient and reliable amounts has not been satisfactory, and can not be used in the treatment of pathological, cellular repair and survival, etc., to enhance the healing of damaged tissue, improve wound healing or tissue regeneration, and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 3',5'-Diacyl-2'-deoxythymidine from acid anhydrides
[0158] 2'-Deoxythymidine is dissolved in anhydrous pyridine at room temperature. 2.1 molar equivalents of the acid anhydride of the desired acyl compound (e.g., acetic anhydride, lactate anhydride, butyric anhydride, etc.) is then added. The reaction mixture is then heated to 80-85.degree. C. for 1 to 4 hours, cooled, poured into ice water, and the esters recovered by extraction with chloroform or a similar solvent. The chloroform is then washed with ice-cold 0.01 N sulfuric acid, 1% aqueous sodium bicarbonate, and finally water. After drying with sodium sulfate, the chloroform is evaporated and the residual oil or crystals are subjected to chromatography (adapted from Nishizawa et al., Biochem. Pharmacol. 14:1605 (1965)).
[0159] From Acid Chlorides:
[0160] To 2'-deoxythymidine dissolved in anhydrous pyridine is added, at 5.degree. C., 2.1 molar equivalents of the acid chloride of the desired acyl compound (e.g., palmit...
example 2
Preparation of 5'-Acyl-2'-deoxythymidine
[0161] To 2'-deoxythymidine dissolved in anhydrous pyridine is added, at room temperature, 1.0 molar equivalent of the acid anhydride of the desired acyl compound. The reaction is then heated to approximately 80-85.degree. C. for several hours, cooled, poured into ice water, and the esters recovered by extraction with chloroform or a similar solvent. The chloroform is then washed in ice-cold 0.01 N sulfuric acid, 1% aqueous sodium bicarbonate, and finally water. After drying with sodium sulfate, the chloroform is evaporated and the residual oil or crystals are subjected to chromatography. The major product, which is isolated by chromatography is the 5' substituted ester (adapted from Nishizawa et al.
[0162] Alternatively, selectively 5' acylation of deoxythymidine may be accomplished by suspending 2'-deoxythymidine in a mixture of pyridine and N,N-dimethylformamide cooled to 0.degree. C. in an ice bath. 1.0 molar equivalent of the acid chloride...
example 3
Preparation of 3'-Acyl-2'-deoxythymidine
[0163] To a stirred suspension of 2'-deoxythymidine in dry N,N-dimethylformamide is added 2.4 molar equivalents of imidazole followed by 1.2 molar equivalents of t-butyldimethylchlorosilane. The mixture is stirred with protection from moisture at room temperature for 20 hours, at which time the solvent is removed at 50.degree. C. in vacuo. The residue is dissolved in 15 ml of ethyl acetate, washed, and evaporated to give a syrup from which is obtained, by crystallization from hot chloroform by the addition of hexane to the point of opalescence, 5'-(t-butyldimethylsilyl)-2'-deoxythymidine.
[0164] To a stirred suspension of 5'-(t-butylmethylsilyl)-2'-deoxythymidin-e in dry pyridine cooled to 0.degree. C. is added 1.1 molar equivalents of the appropriate acid anhydride of the desired acyl compound, and the mixture is stirred with protection from moisture for 20 hours at 0-5.degree. C., at which time the reaction is terminated by addition of a few ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com