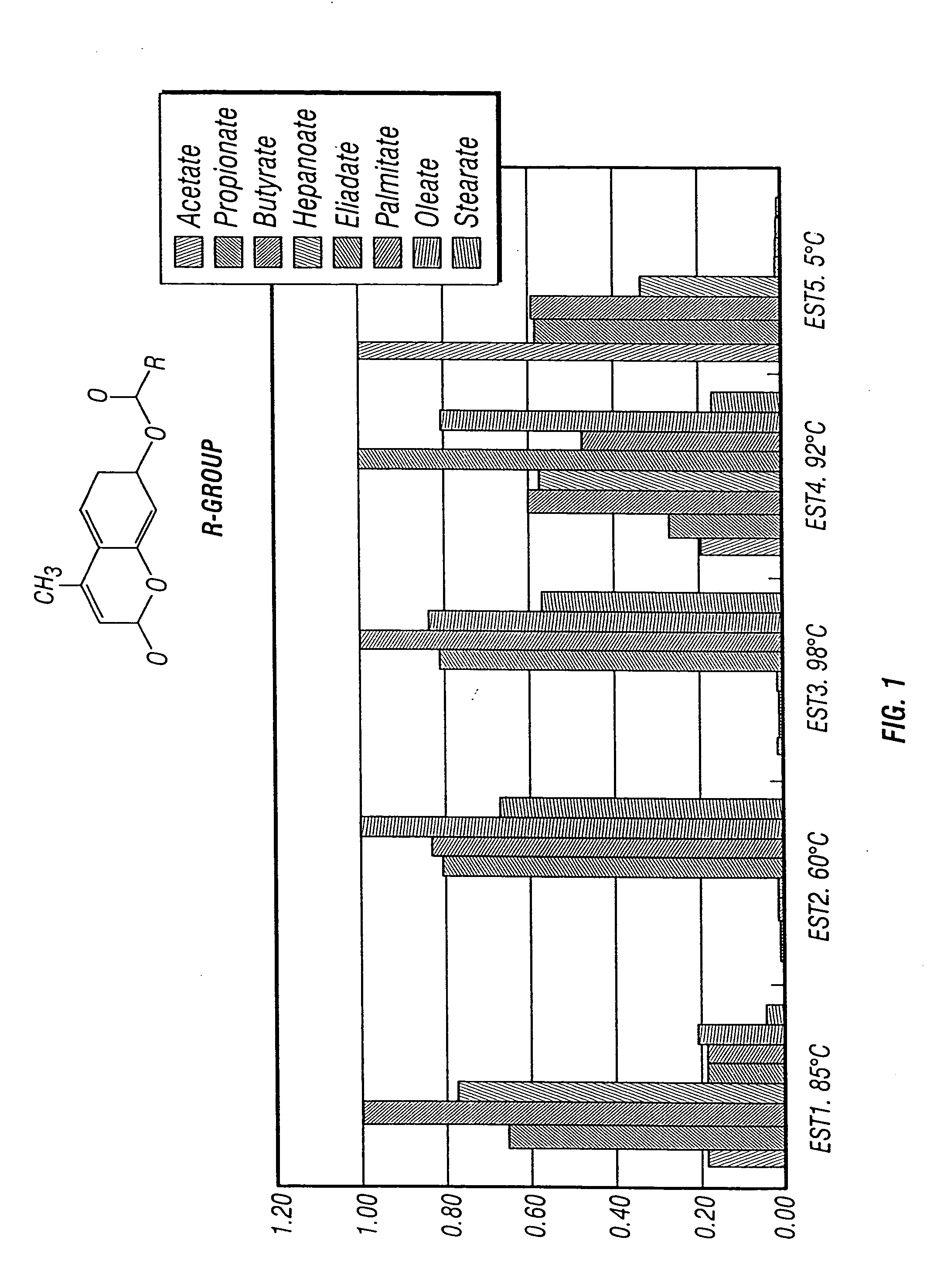
High throughput screening for novel bioactivities
a bioactivity and high throughput technology, applied in the field of identification of new bioactivities, can solve the problems of difficult chemical duplicate, low enantioselectivity, and hampered synthesis of polymers,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
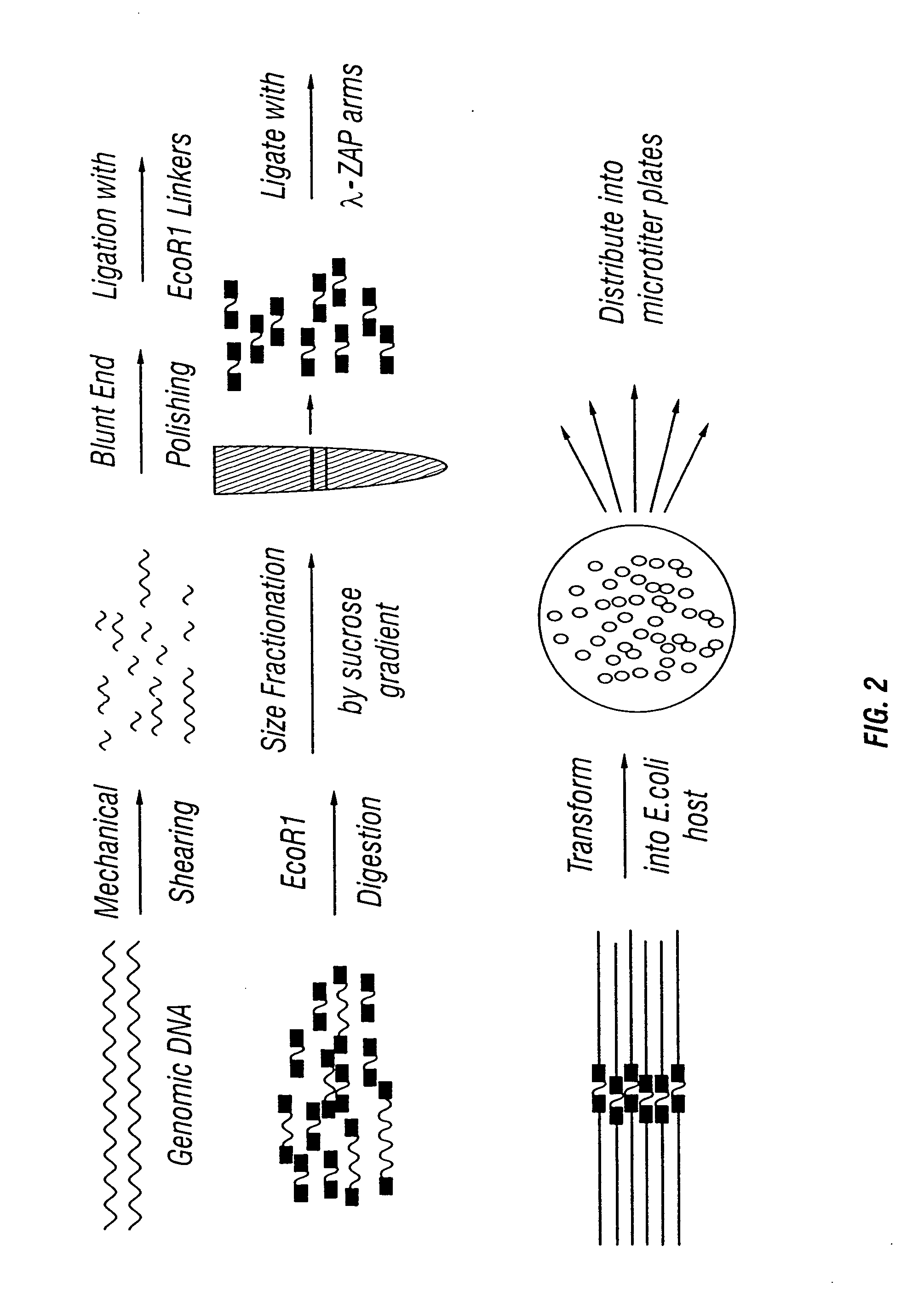
DNA Isolation and Library Construction
[0218] The following outlines the procedures used to generate a gene library from an environmental sample.
[0219] DNA isolation. DNA is isolated using the IsoQuick Procedure as per manufacturer's instructions (Orca, Research Inc., Bothell, Wash.). DNA can be normalized according to Example 2 below. Upon isolation the DNA is sheared by pushing and pulling the DNA through a 25 G double-hub needle and a 1-cc syringes about 500 times. A small amount is run on a 0.8% agarose gel to make sure the majority of the DNA is in the desired size range (about 3-6 kb).
[0220] Blunt-ending DNA. The DNA is blunt-ended by mixing 45 μl of 10× Mung Bean Buffer, 2.0 T1 Mung Bean Nuclease (150 u / TI) and water to a final volume of 405 T1. The mixture is incubate at 37° C. for 15 minutes. The mixture is phenol / chloroform extracted followed by an additional chloroform extraction. One ml of ice cold ethanol is added to the final extract to precipitate the DNA. The DNA i...
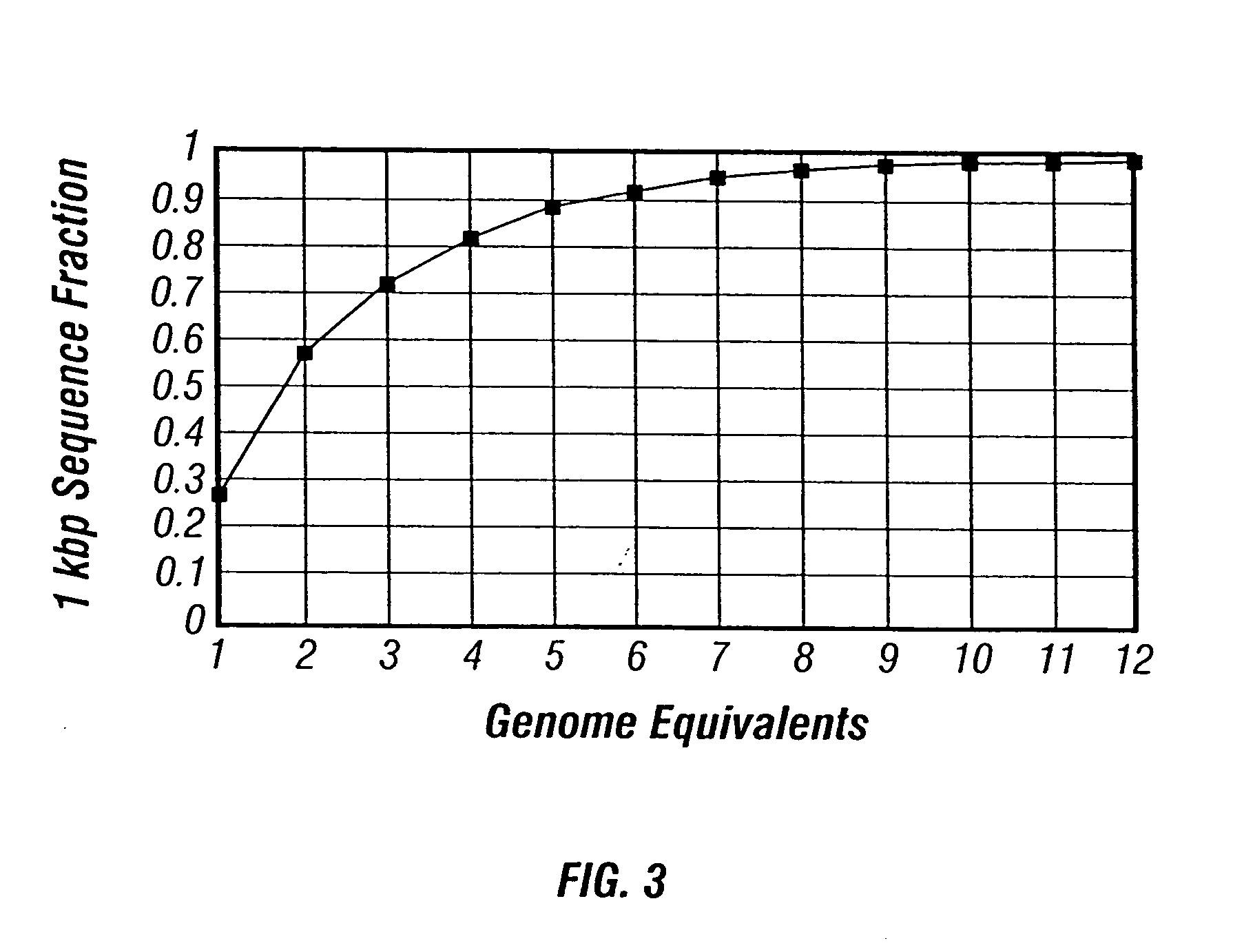
example 2
Normalization
[0234] Prior to library generation, purified DNA can be normalized. DNA is first fractionated according to the following protocol. A sample composed of genomic DNA is purified on a cesium-chloride gradient. The cesium chloride (Rf=1.3980) solution is filtered through a 0.2 Tm filter and 15 ml is loaded into a 35 ml OptiSeal tube (Beckman). The DNA is added and thoroughly mixed. Ten micrograms of bis-benzimide (Sigma; Hoechst 33258) is added and mixed thoroughly. The tube is then filled with the filtered cesium chloride solution and spun in a VTi50 rotor in a Beckman L8-70 Ultracentrifuge at 33,000 rpm for 72 hours. Following centrifugation, a syringe pump and fractionator (Brandel Model 186) are used to drive the gradient through an ISCO UA-5 UV absorbance detector set to 280 nm. Peaks representing the DNA from the organisms present in an environmental sample are obtained. Eubacterial sequences can be detected by PCR amplification of DNA encoding rRNA from a 10-fold di...
example 3
Cell Staining Prior to FACS Screening
[0237] Gene libraries, including those generated as described in Example 1, can be screened for bioactivities of interest on a FACS machine as indicated herein. A screening process begins with staining of the cells with a desirable substrate according to the following example.
[0238] A gene library is made from the hyperthermophilic archaeon Sulfulobus solfataricus in the Σ-ZAPII vector according to the manufacturers instructions (Stratagene Cloning Systems, Inc., La Jolla, Calif.), and excised into the pBLUESCRIPT plasmid according to the manufacturers instructions (Stratagene). DNA was isolated using the IsoQuick DNA isolation kit according to the manufacturers instructions (Orca, Inc., Bothell, Wash.).
[0239] To screen for θ-galactosidase activity, cells are stained as follows. Cells are cultivated overnight at 37° C. in an orbital shaker at 250 rpm. Cells are centrifuged to collect about 2×107 cells (0.1 ml of the culture), resuspended in 1 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com