Stable, cationically polymerizable/crosslinkable dental compositions having high filler contents
a dental composition and polymerization technology, applied in dental prosthetics, dental preparations, applications, etc., can solve the problems of affecting the mechanical reinforcement function assigned, the filler level of such compositions rarely exceeds 45%, and the absorption capacity of resin is limited, so as to achieve economic viability, easy to implement, and substantial stability of compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1.1 Preparation of a Control Formulation 1 without Dispersant:
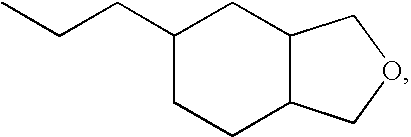
A Hauschild® centrifuge mixer is charged with 25 g of quartz (SiO2>99%) with a particle size of 5 μm, sold by Schott, 3 g of ytterbium trifluoride, 10 g of siloxane resin having a monomer (A) content >90%, obtained by hydrosilylating VCMX according to a preparation process as described above.
Stirring is carried out for 16 s with the centrifuge mixer and then 1.25 g of photoinitiator system are added, containing 30% of photoinitiator P1 and 0.23% of a photosensitizer based on chloropropoxythioxanthone CPTX, sold by Lambson, all in solution in the resin (A) without solvent. Stirring is carried out for 16 s with the centrifuge mixer. Then 5 g of fumed silica (SiO2>99%) are added and the mixture is stirred for 16 s. 5.75 g of fumed silica are added and the mixture is then stirred for 16 s.
The fumed silica is a silica sold by Degussa under the name OX-50, with a specific surface area of 40 m2 / g.
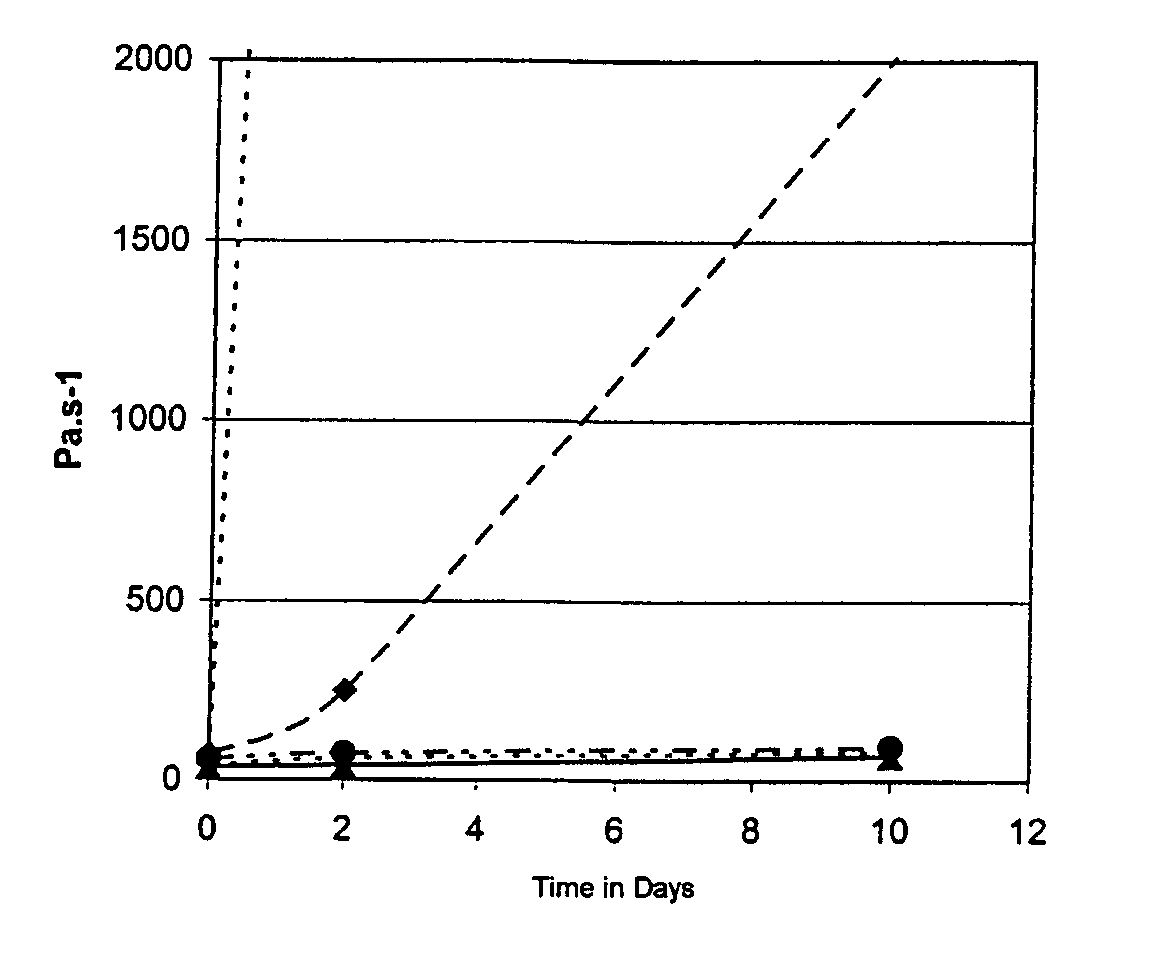
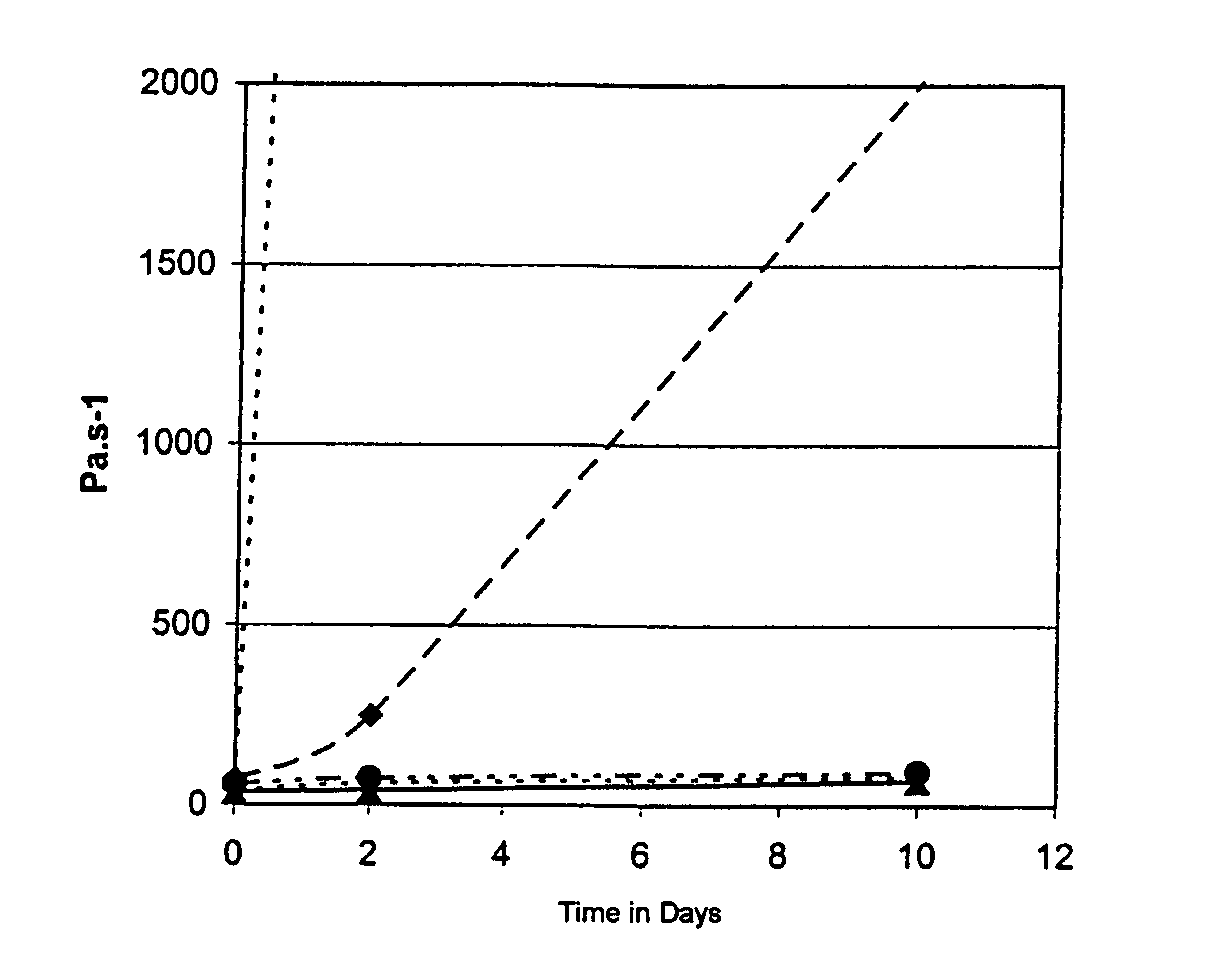
The curve of change in ...
example 2
2.1. Preparation According to the Invention:
The dispersant Byk® 164 (8 g) is dissolved in the resin (A) at 4% and the solution is subsequently devolatilized so as to remove the butyl acetate present in the dispersant, by heating under a vacuum of 10 mmHg at 60° C. for 3 hours in a rotary evaporator.
The active substance concentration is 2.4%.
2.2 Preparation of a Formulation 1 with Dispersant:
A Hauschild® centrifuge mixer is charged with 25 g of quartz (SiO2>99%) with a particle size of 5 μm, sold by Schott, 3 g of ytterbium trifluoride, 1.25 g of the solution of dispersant Byk® 164 devolatilized in the resin (A) as described above, and stirring is carried out for 16 s with the centrifuge mixer. 9 g of resin (A) are added.
Stirring is carried out for 16 s with the centrifuge mixer and then 1.25 g of photoinitiator system in (A) are added, containing 30% of photoinitiator P1 and 0.23% of photosensitizer CPTX. Stirring is carried out for 16 s with the centrifuge mixer. Then 5 ...
example 3
Photopolymerization of Dental Restoration Formulations 1, 2 and 3 (§ 2.2, 2.3 and 2.4 Above):
The formulations crosslink over a thickness of 3 mm in 40 of irradiation with an Optilux® Demetron lamp. The Vickers hardness measured after photocrosslinking is 50 for each of the three formulations. The flexural modulus and flexural strength measured in accordance with standard ISO4049 are 5 GPa and 80 MPa respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| acid index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com