Electrochemical synthesis of ammonia
a technology of ammonia and electrochemical synthesis, which is applied in the direction of electrolysis process, electrolysis components, etc., can solve the problem of reducing the efficiency of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
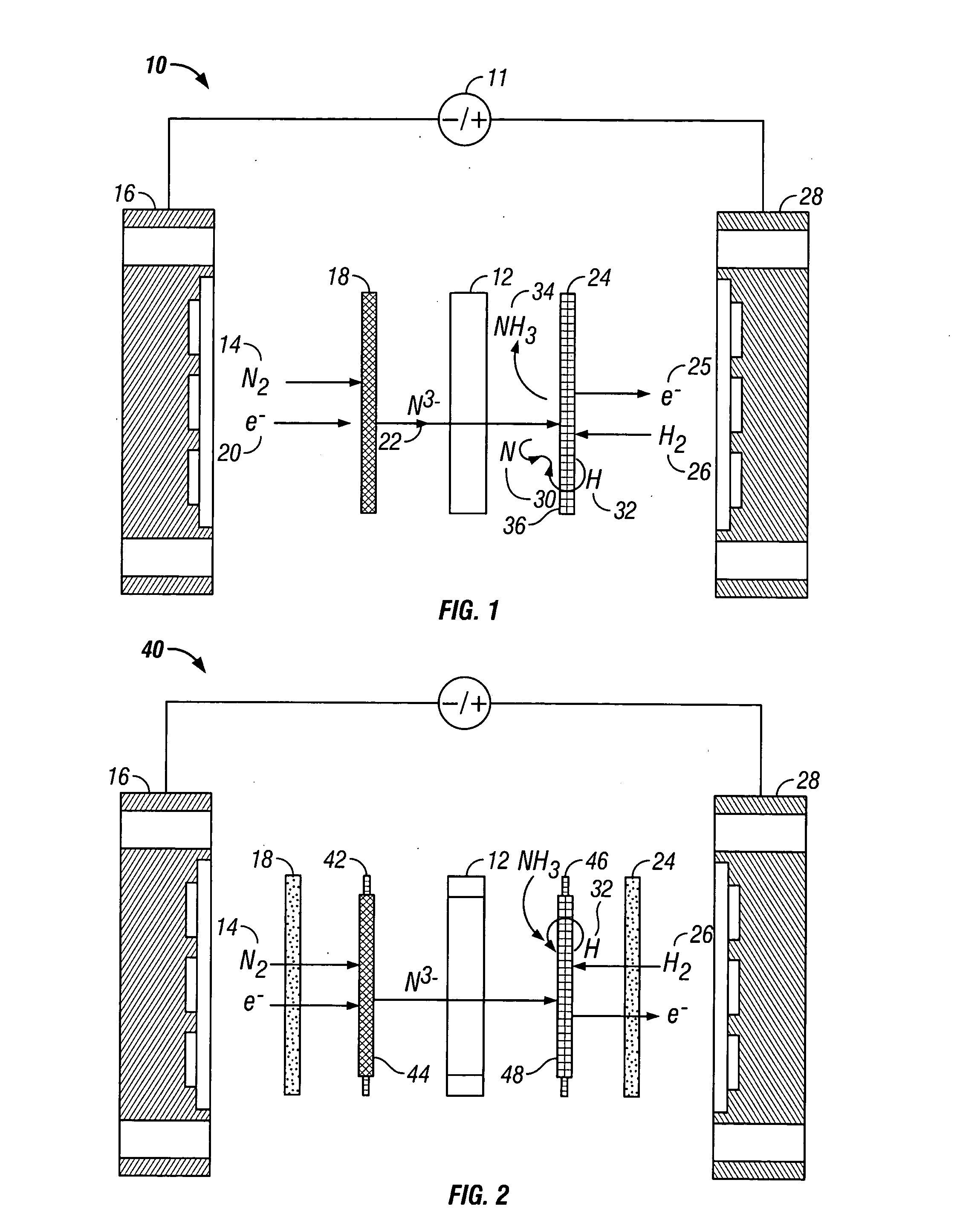
[0056] Anhydrous lithium chloride and potassium chloride (Sigma Aldrich, St. Louis, Mo.) was vacuum dried for 48 hours at 140° C. After drying, the powders were removed from the vacuum oven and immediately placed into a vacuum dessicator before being transferred to a Vacuum Atmosphere Company dry box. An argon atmosphere was maintained at all times in the dry box, with oxygen and moisture concentrations below the detection limit of the sensors (1 ppm). A 59% LiCl / 41% KCL / 0.1% Li3N molar salt mixture was prepared by grinding the salts together with mortar and pestle, before transferring to a 100 ml high form alumina crucible (Fisher Scientific, Pittsburgh, Pa.).
[0057] All electrochemical measurements were performed versus a lithium / lithium ion reference electrode. The electrochemical cell was assembled in the glove box with the fuel cell type anode and cathode electrodes positioned with the active sides facing each other. The cathode was a sintered nickel gas diffusion electrode and...
example 2
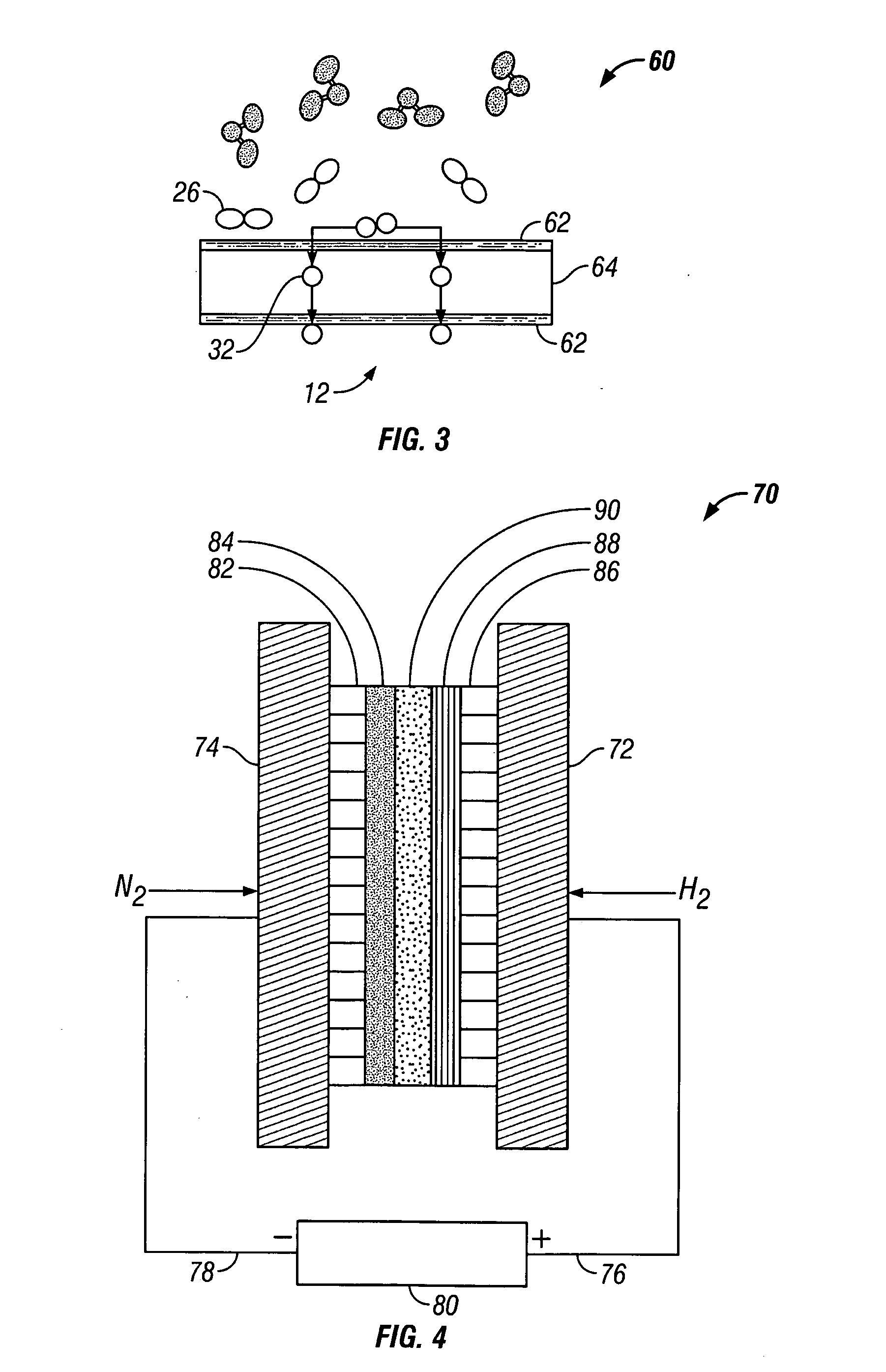
[0068] An electrolyte salt mixture was prepared as described in Example 1 in a high form crucible. A fuel cell type cathode having a sintered nickel face was used along with an anode made from a titanium sheet attached to a nickel wire. Both electrodes were sealed into the reactor cap, placed into the powdered electrolyte salt. The reactor cap was then secured in position and sealed and an inert atmosphere was maintained. The reactor was then placed into a heater unit. Both electrodes were attached to a Tenmax Laboratory DC Power Supply Model No. 72-420. The nitrogen gas used to generate the nitride ion was dried using molecular sieves before it was flowed into the cell. Once a gas flow was established, the system was back pressured using a valve on the outlet line to prevent the molten salt from filling the internal cavity. Once the eutectic salt mixture reached the melting temperature a constant current of 0.1 Amp was applied for 45 minutes. As the reaction proceeded, the cell vol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com