Immunisation against chlamydia trachomatis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
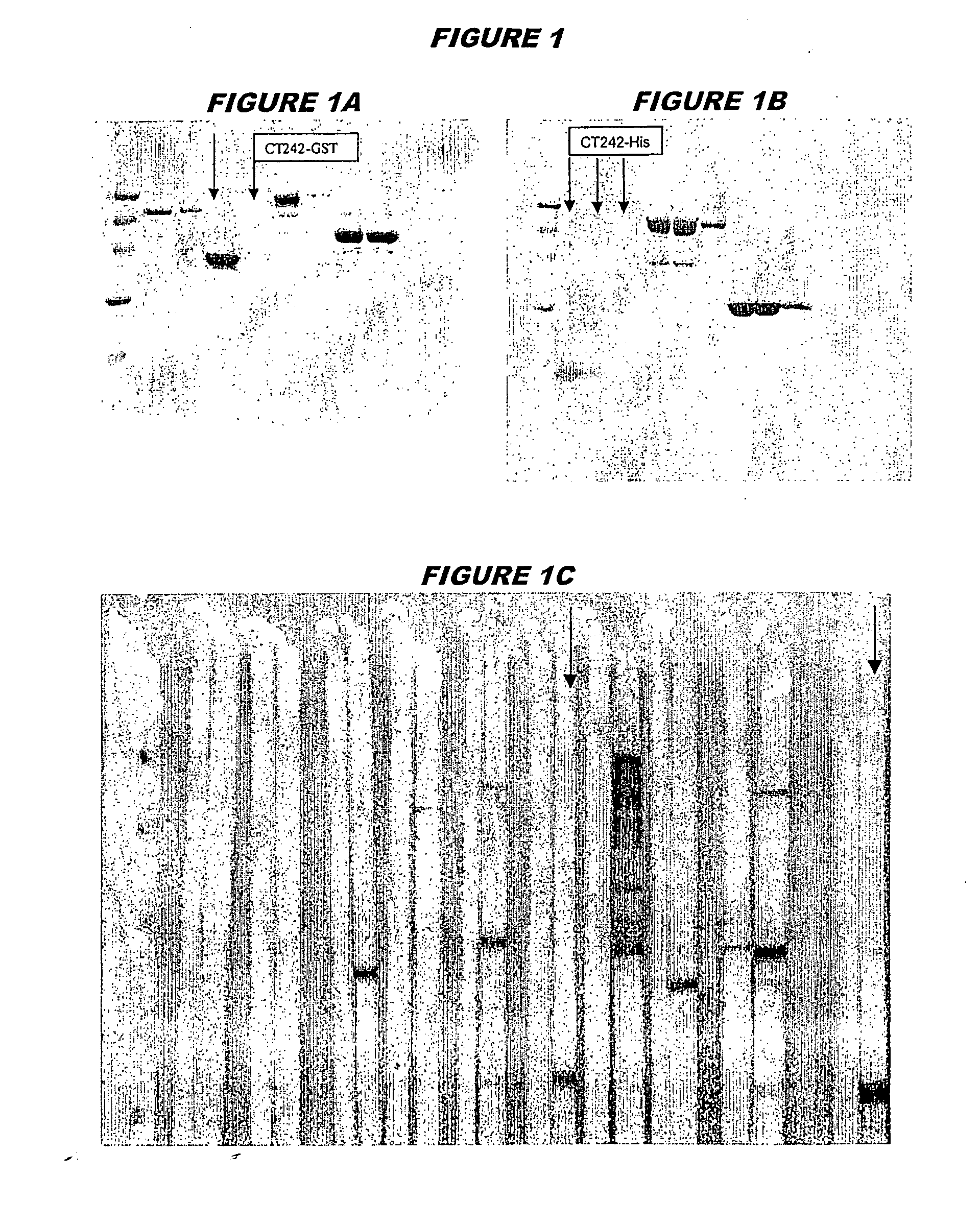
[0236] CT242 (SEQ ID 57 and SEQ ID 58) was expressed in E.coli. The recombinant product was purified both as a GST-fusion protein (FIG. 1A; lanes 4 and 5, chromatography fractions 1 and 2, expected molecular weight 42.4 kDa) and as a His-tagged fusion protein (FIG. 1B; lanes 2-4, chromatography fractions 1, 2 and 3, expected molecular weight 16.4 kDa).
[0237] The recombinant protein was used to immunise mice, whose sera were used in a Western blot (FIG. 1C: His-tagged: lanes 12 and 13; GST-fusion: lanes 20 and 21). Lane 12 shows membrane strips stained with pre-immune sera for His-tagged CT242 whilst lane 13 shows membrane strips stained with immune sera for His-tagged CT242. Lane 20 shows membrane strips stained with preimmune sera for GST-fusion CT242 whilst lane 21 shows membrane strips stained with immune sera for GST-fusion CT242.
[0238] These experiments show that CT242 is a surface-exposed and immunoaccessible protein, and that it is a useful immunogen. These properties are n...
example 2
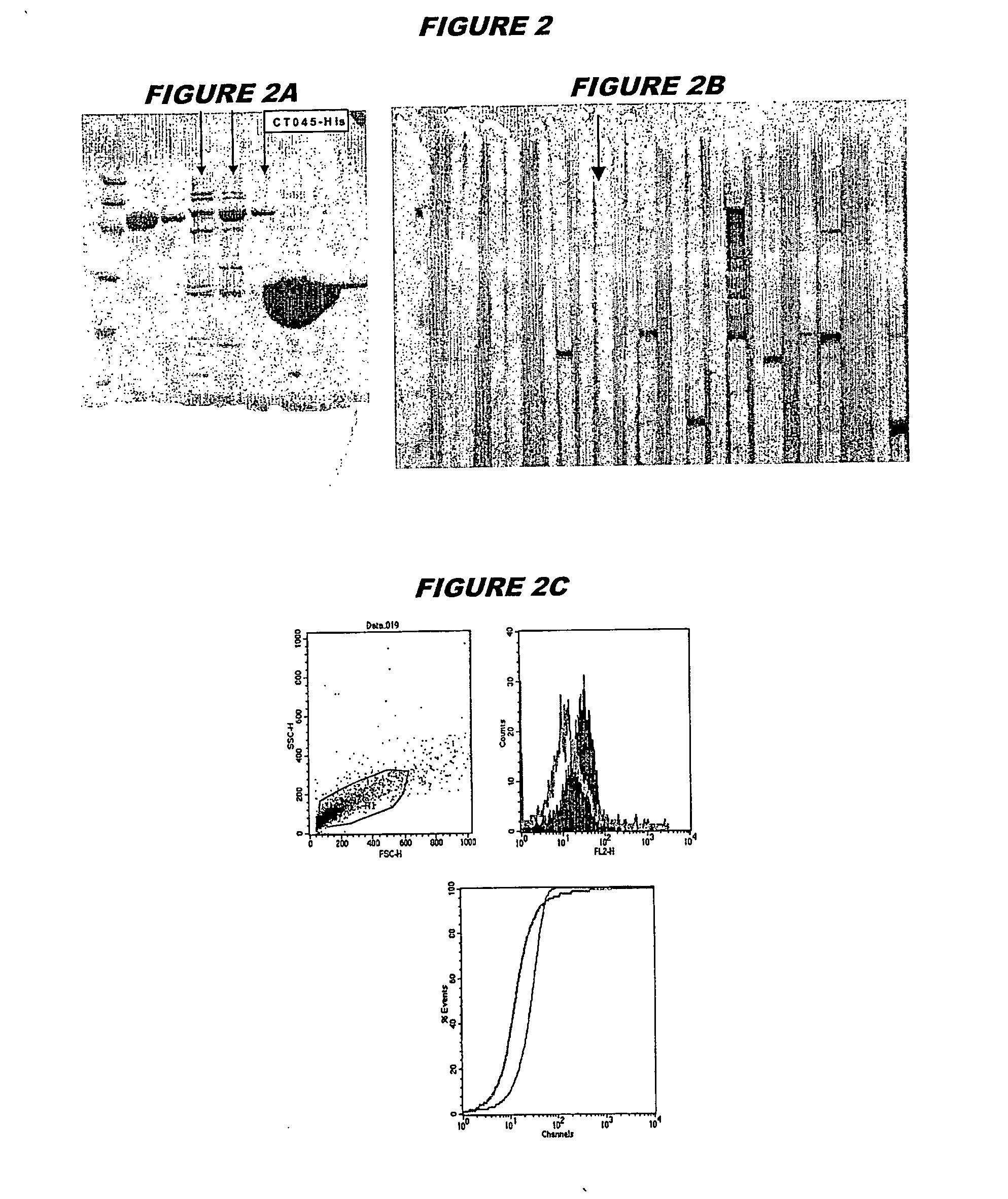
[0239] CT045 (SEQ ID 71 and SEQ ID 72) was expressed in E.coli. The recombinant product was purified as a His-tagged fusion protein (FIG. 2A; lanes 4-6, chromatography fractions 1, 2 and 3, expected molecular weight 55.8 kDa). The recombinant protein was used to immunise mice, whose sera were used in a Western blot (FIG. 2B, lanes 8 and 9) and for FACS analysis (FIG. 2C, K-S value 16.81).
[0240] These experiments show that CT045 is a surface-exposed and immunoaccessible protein, and that it is a useful immunogen. These properties are not evident from the sequence alone.
example 3
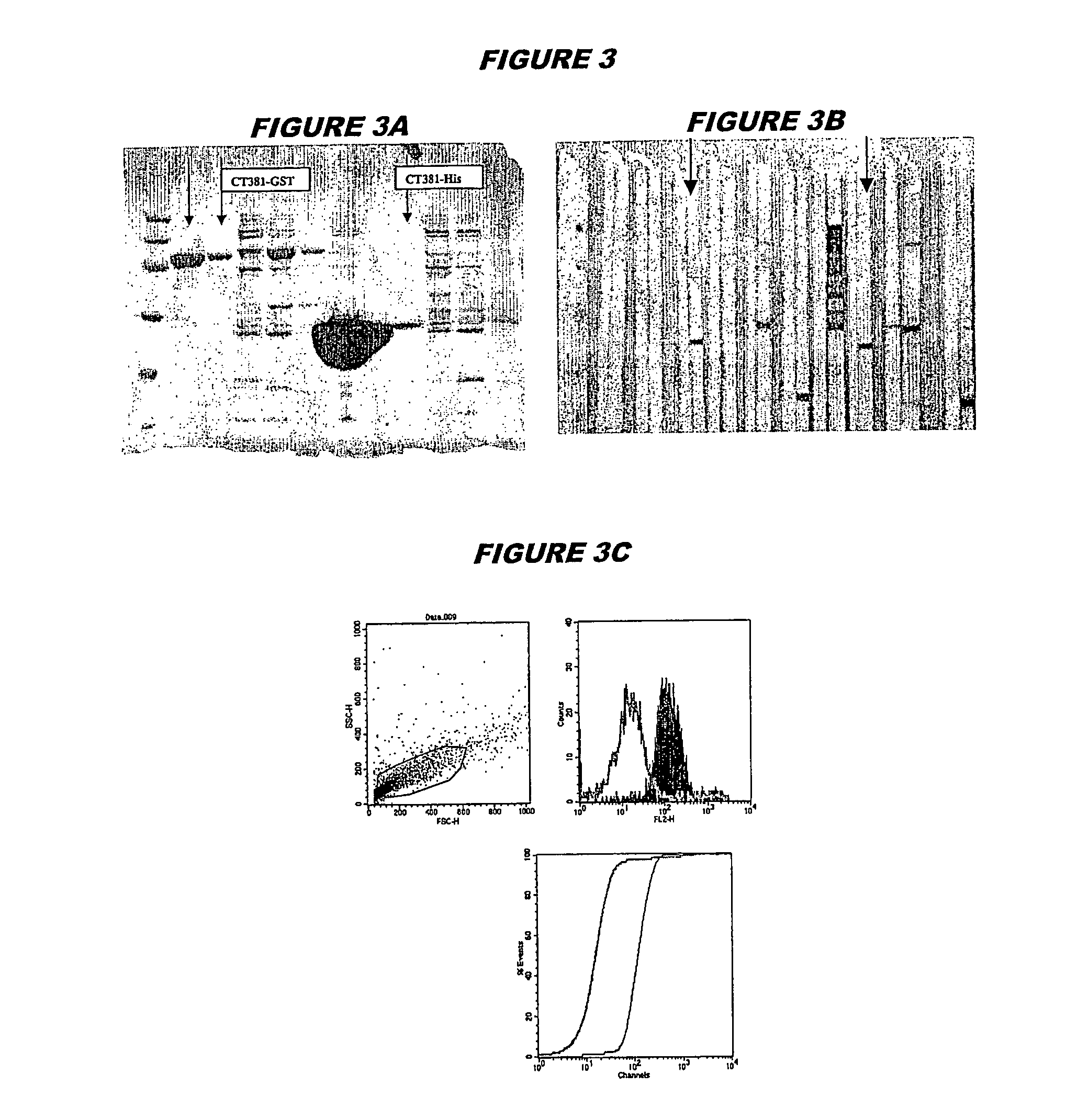
[0241] CT381 (SEQ ID 105 and SEQ ID 106) was expressed in E.coli. The recombinant product was purified both as a GST-fusion protein (FIG. 3A; lanes 2 and 3, chromatography fractions 1 and 2, expected molecular weight 52.7 kDa) and as a His-tagged fusion protein (FIG. 3A; lanes 7-9, chromatography fractions 1, 2 and 3, expected molecular weight 26.7 kDa). The recombinant protein was used to immunise mice, whose sera were used in a Western blot (FIG. 3B: His-tagged: lanes 6 and 7; GST-fusion: lanes 16 and 17) and for FACS analysis (FIG. 3C: GST-tagged, K-S value 35.98; FIG. 3D: His-tagged, K-S value 32.54).
[0242] These experiments show that CT381 is a surface-exposed and immunoaccessible protein, and that it is a useful immunogen. These properties are not evident from the sequence alone.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com