Method for amplifying nucleic acid sequence
a nucleic acid sequence and amplifying technology, applied in the field of amplifying nucleic acid sequence, can solve the problems of serious running cost associated with the use of modified deoxyribonucleotide triphosphate, the use of expensive thermal cyclers, and the cleavability of amplified dna fragments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
referential example 1
Preparation of RNase H from Thermophile Bacillus caldotenax
[0437]Bacillus caldotenax YT-G (purchased from Deutsche Sammlung von Mikroorganismen; DSM406) was inoculated into 100 ml of a medium containing 0.2% Tryptone (Difco Laboratories) and 1.5% yeast extract (Difco Laboratories) (pH 6.5), cultured at 60° C. for 140 minutes with shaking and used as a pre-culture. 30 ml of the pre-culture was inoculated into 3 L of a medium having the same composition and cultured with aeration at 2.5 L / minute and stirring at 250 rpm at a temperature of 60° C. for 5 hours.
[0438] The cells were collected by centrifuging the culture at 5000×g for 15 minutes. 402 g (wet weight) of the cells were suspended in 1000 ml of 50 mM tris-HCl buffer (pH 7.5) containing 10 mM mercaptoethanol, 0.5 M NaCl, 1 mM EDTA and 20 μM PAPMSF and disrupted using MINI-Lab (APV GAULIN / RANNIE). Cell debris were removed by centrifugation to recover a supernatant.
[0439] A polyethylene imine solution was added to the resulting...
referential example 2
Cloning of Bacillus caldotenax RNase HII Gene
[0447] (1) Preparation of Genomic DNA from Bacillus caldotenax
[0448]Bacillus caldotenax YT-G (DSM406) was inoculated into 60 ml of LB medium (1% Tryptone, 0.5% yeast extract and 0.5% NaCl, pH 7.2) and cultured at 65° C. for 20 hours. After culturing, the culture was centrifuged to collect cells. The cells were suspended in 2 ml of 25% sucrose and 50 mM tris-HCl (pH 8.0). 0.2 ml of 10 mg / ml lysozyme chloride (Nacalai Tesque) in water was added thereto. The mixture was reacted at 20° C. for 1 hour. After reaction, 12 ml of a mixture containing 150 mM NaCl, 1 mM EDTA and 20 mM tris-HCl (pH 8.0), 0.1 ml of 20 mg / ml proteinase K (Takara Shuzo) and 1 ml of a 10% aqueous solution of sodium lauryl sulfate were added to the reaction mixture. The mixture was incubated at 37° C. for 1 hour.
[0449] 2.1 ml of 5 M NaCl and 2 ml of a CTAB-NaCl solution [10% cetyltrimethylammonium bromide (Nacalai Tesque) and 0.7 M NaCl] were then added to the mixture ...
example 2-(
3)
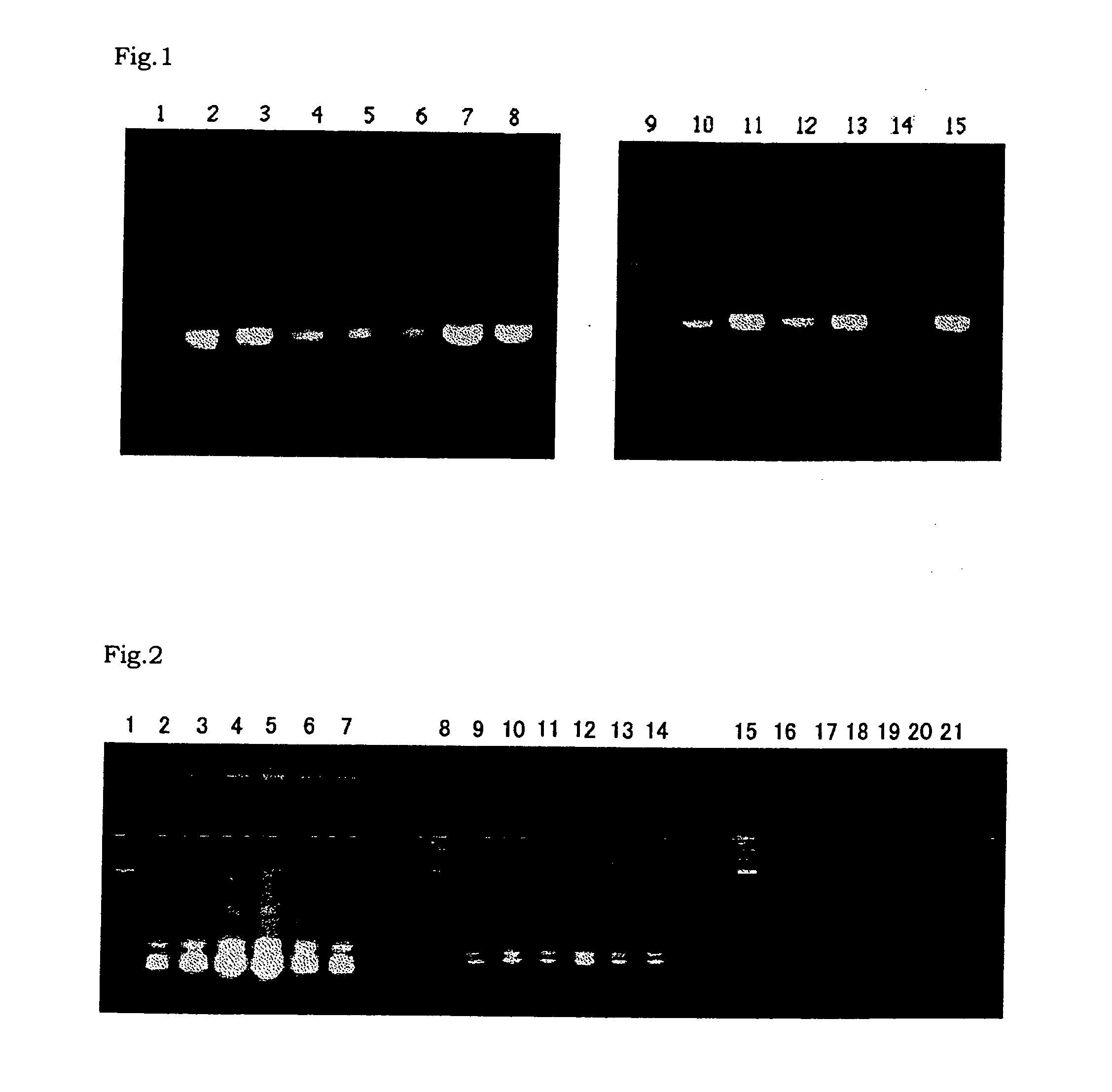
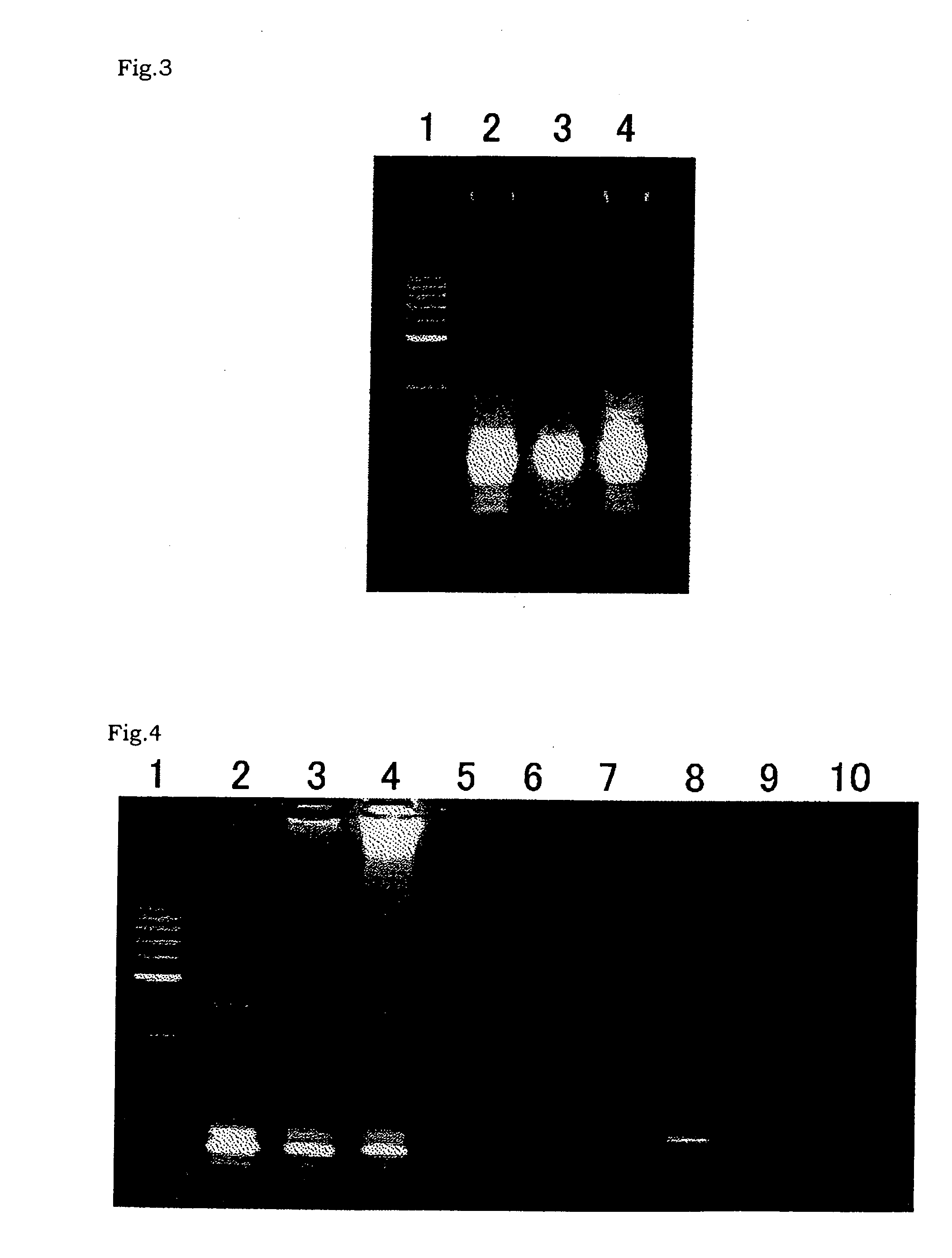
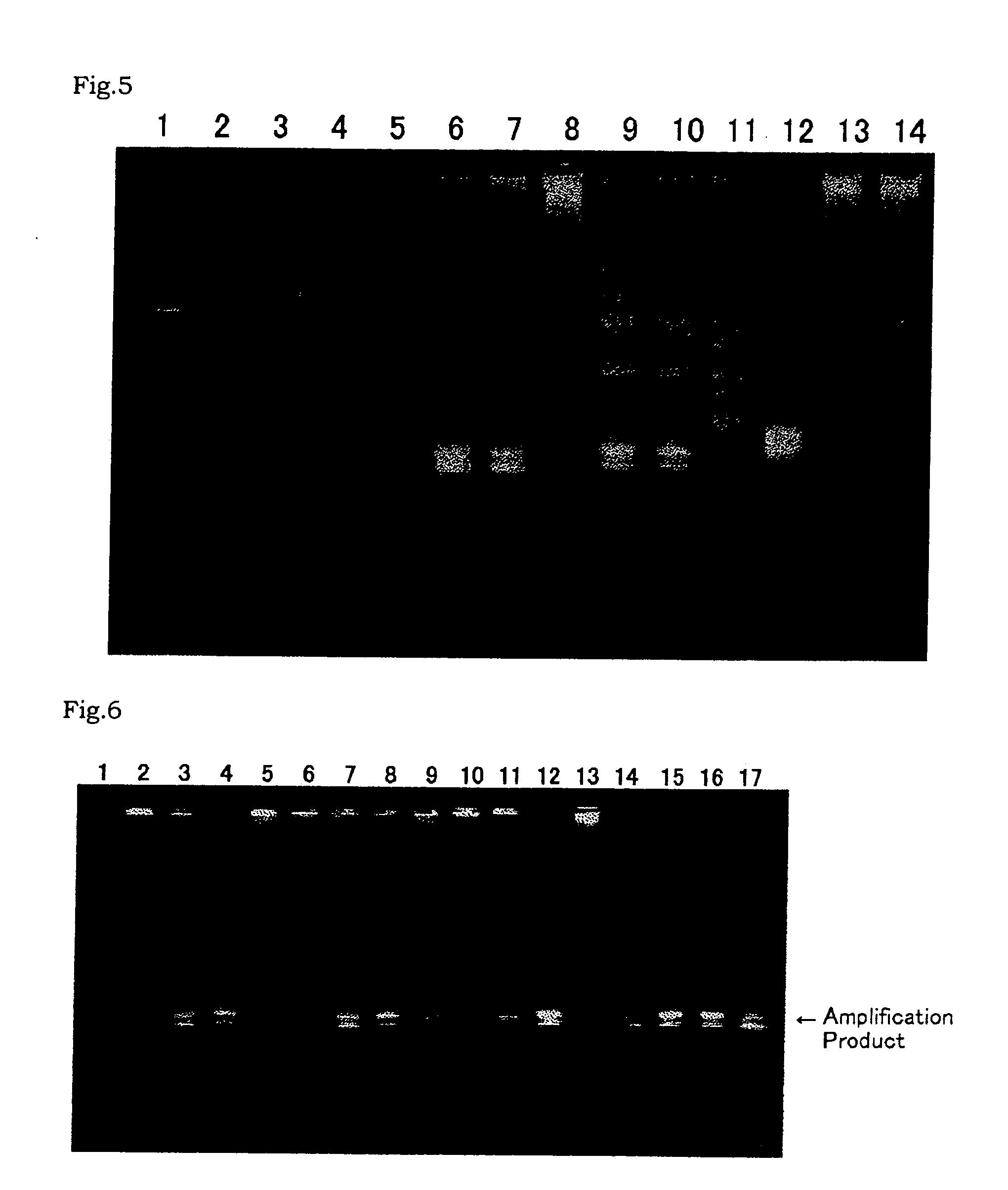
[0459] A PCR was carried out using the plasmid 21-12 as prepared in Referential Example 2-(2) as a template, and RNII-S5 and RNII-S6 as primers. TaKaRa Ex Taq polymerase (Takara Shuzo) was used as a DNA polymerase for the PCR according to the attached protocol. The PCR was carried out as follows: 25 cycles of 94° C. for 30 seconds, 55° C. for 30 seconds and 72° C. for 30 seconds. After reaction, the reaction mixture was subjected to agarose gel electrophoresis. An amplified DNA fragment of about 0.3 kb was recovered from the gel. The about 0.3-kb DNA fragment was labeled with digoxigenin using DIG High-prime (Roche Diagnostics).
[0460] Southern Hybridization was carried out for digests of the Bacillus caldotenax genomic DNA as prepared in Referential Example 2-(1) with HindIII (Takara Shuzo), Sac I (Takara Shuzo), or HindIII and SacI using the digoxigenin-labeled DNA as a probe. Hybridization and detection were carried out using DIG Luminescent Detection Kit (Roche Diagnostics) ac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| optimal growth temperature | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com