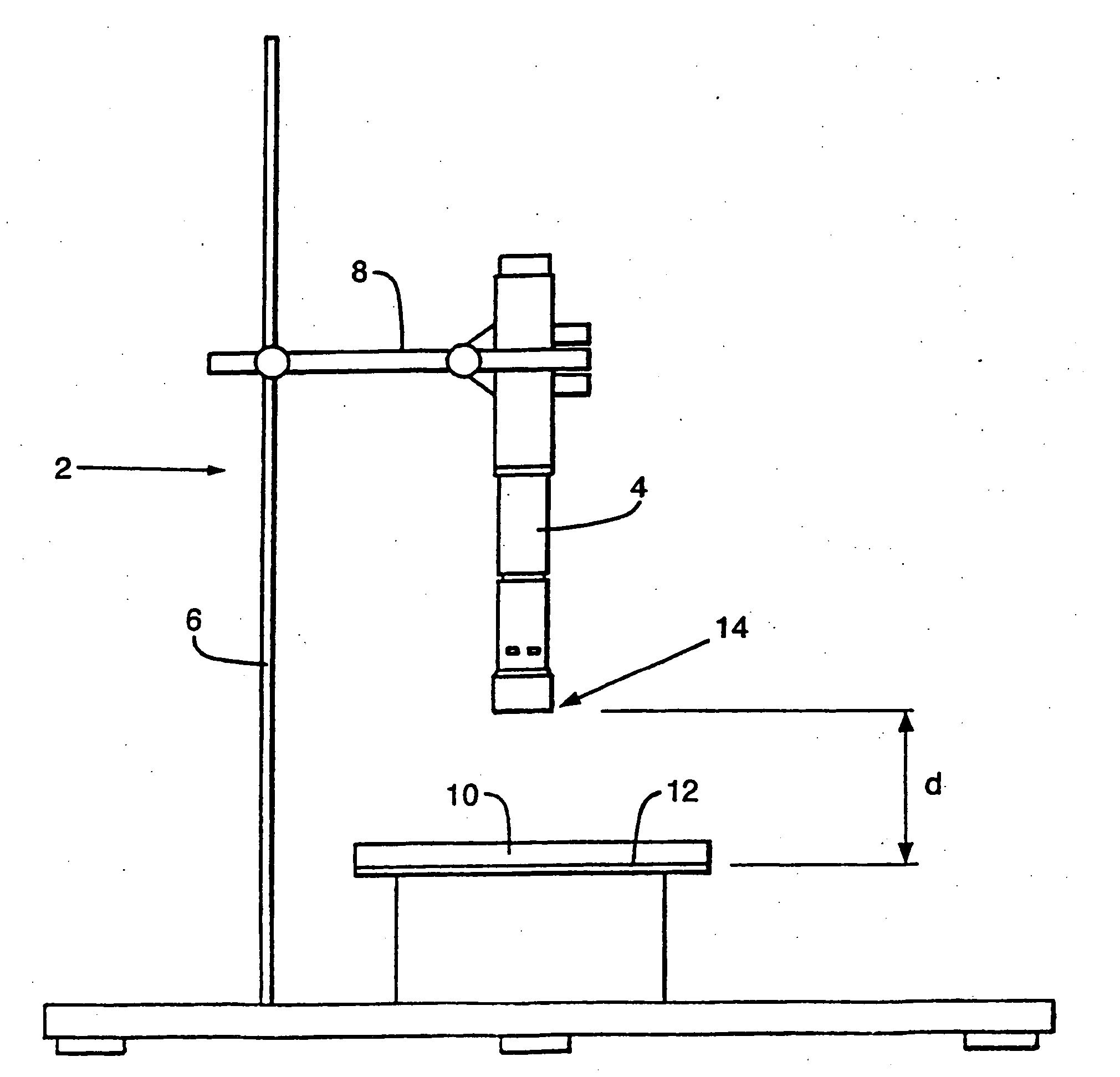
Particle delivery techniques
a technology of particle delivery and delivery method, which is applied in the direction of microinjection, biochemistry apparatus and processes, and genetic material ingredients, etc., can solve the problems of unfavorable tissue specific delivery, and the inability to safely and effectively administer drugs using traditional transdermal delivery methods, etc., to achieve optimal tissue-specific delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
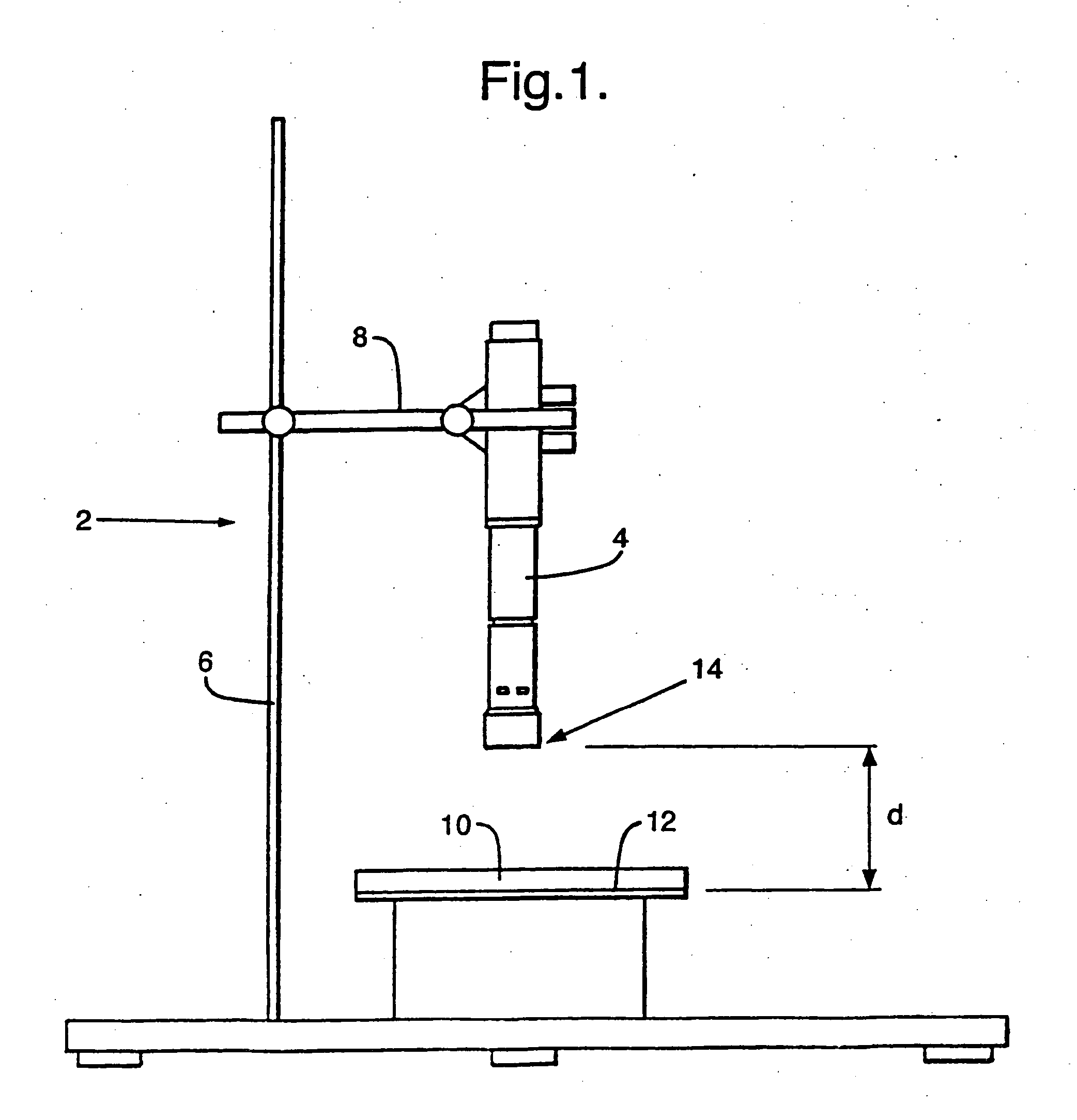
Image
Examples
example 1
[0107] The following experiment was conducted to investigate the possibility of using freeze-dried DNA as an alternative to DNA-coated metal particles in the biolistic transfer of genetic material. In particular, powdered DNA plasmids as well as DNA-coated tungsten particles as controls were delivered ex vivo to male human fibroblast HT1080 cells using a needleless syringe apparatus as follows.
[0108] Clone 123 is a small plasmid of ˜11 kb which contains the β-galactosidase marker gene so that transient transformation can be measured with the chromogenic indicator X-Gal. Plasmids were bulked with a carbohydrate excipient, trehalose. Trehalose was selected as the excipient because of its stabilizing properties (Colaco et al. (1992) Bio / Technology 10:1009). The trehalose was dissolved in distilled water and filter-sterilized prior to adding the DNA to the solution. Three different solutions of DNA sugar were made up with the proportions shown below in Table 1.
TABLE 1Preparation123Cl...
example 2
[0121] The following studies were carried out to assess the ability to deliver a powdered nucleic acid composition to test subjects in vivo using the methods of the invention.
[0122] Plasmid Vector Construct: The pGREEN-1 vector construct, which contains the Green Fluorescent Protein (GFP) gene under the control of a CMV promoter, was used so that gene expression could be assessed directly by UV microscopy of histological sections from treated tissue samples.
[0123] Powdered Nucleic Acid Compositions: A powdered nucleic acid composition was prepared as follows. A mixture was formed by combining pGREEN-1 vector plasmid with trehalose sugar to obtain a 1 μg:1 mg (w / w) DNA-sugar composition. This composition was lyophilized, compressed, ground, and then sieved, using the techniques described hereinabove. The resulting condensed nucleic acid composition had an average particle size ranging from about 38-75 μm.
[0124] Administrations: C57BL / 10 mice were treated with 1 mg of the particula...
example 3
Densification of Recombinant Human Growth Hormone (rhGH)
[0127] Lyophilized recombinant human growth hormone powder (Genotropin®, available from Pharmacia, Piscataway, N.J.) was obtained and reprocessed using the method of the invention. Particularly, approximately 30 mg of Genotropin was compacted under pressure using a Carver Laboratory Pellet Press (Model 3620, available from Carver, Inc., Wabash, Ind.). The pressure of compaction was 15,000 lbs / in2, which was applied for approximately 45 seconds. A pellet was obtained which was ground using mortar and pestle until visually broken up. The resulting reduced pellet was then sieved using a 53 μm sieve (Endecott, London). Particles having a size greater than 53 μm were selected and appropriate dosages thereof were measured into drug cassettes for delivery from a needleless syringe.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com