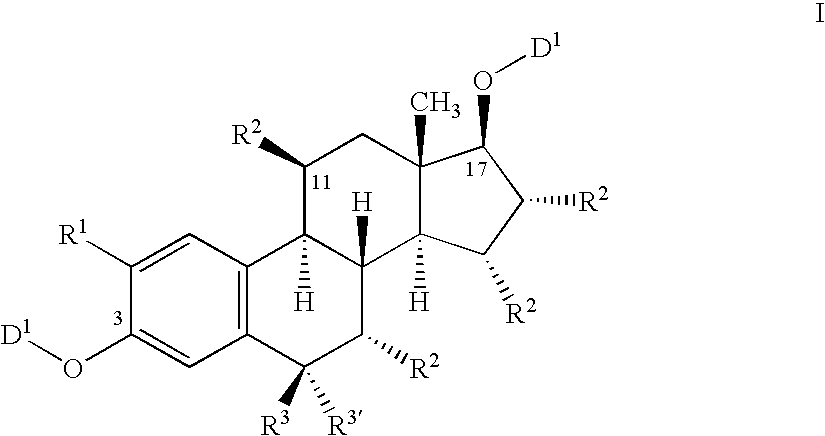
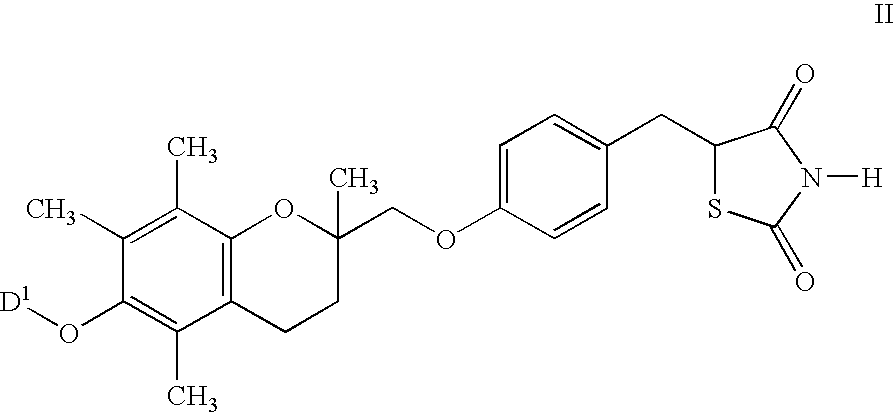
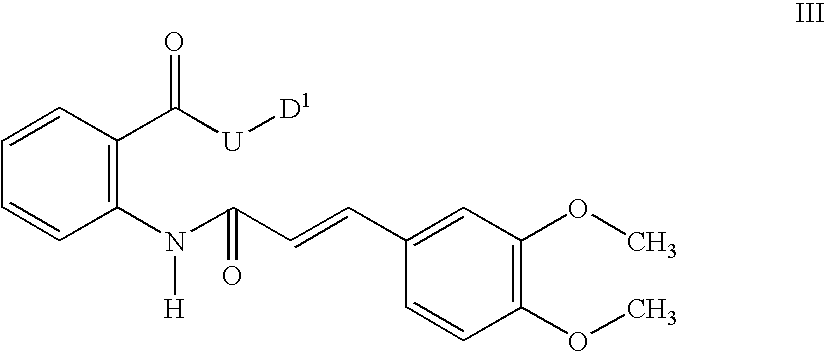
Nitrosated and nitrosylated compounds, compositions and methods use
a technology of nitrosated and nitrosylated compounds, applied in the field of new materials, can solve the problems of no material developed that matches the blood-compatible surface of the endothelium, no synthetic material created that is free of this effect, and the use of anticoagulant and platelet inhibition agents has been less than satisfactory in preventing adverse effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(N-(2-Methyl-2-(nitrosothio)propyl)carbamoyl)methyl 2-((2E)-3-(3,4-dimethoxyphenyl)prop-2-enoylamino)benzoate
[0467]
1a. 2-((2E)-3-(3,4-Dimethoxyphenyl)prop-2-enoylamino)benzoic acid
[0468] The title compound was prepared from 3,4-dimethoxycinnamyl chloride and anthranilic acid according to the procedure in U.S. Pat. No. 3,940,422. 1H NMR (300 MHz, CDCl3 / d6-DMSO) δ 11.62 (s, 1H), 8.84 (d, J=8.5 Hz, 1H), 8.10 (d, J=8.0 Hz, 1H), 7.66 (d, J=15.5 Hz, 1H), 7.55 (t, J=7.7 Hz, 1H), 7.05-7.18 (m, 3H), 6.89 (d, J=8 Hz, 1H), 6.50 (d, J=15.5 Hz, 1H), 3.95 (s, 3H), 3.92 (s, 3H). Mass spectrum (API-TIS) m / z 328 (MH+).
1b. tert-Butyl 2-(2-((2E)-3-(3,4-dimethoxyphenyl)prop-2-enoylamino)phenyl carbonyloxy)acetate
[0469] The product of Example 1a (3.85 g, 11.8 mmol), potassium carbonate (1.62 g, 11.8 mmol) and tert-butyl bromoacetate (1.9 mL, 2.52 g, 13 mmol) in DMF (60 mL) was stirred at room temperature for 4 hours. The reaction mixture was diluted with a large volume of EtOAc, washed several times...
example 2
3-Methyl-3-(nitrosothio)butyl 2-(2-((2E)-3-(3,4-dimethoxyphenyl)prop-2-enoylamino)phenylcarbonyloxy)acetate
[0473]
2a. 3-Methyl-3(2,4,6-trimethoxyphenylmethylthio)butan-1-ol
[0474] To a solution of 3-methyl-3(2,4,6-trimethoxyphenylmethylthio)butyric acid (prepared as described by Lin et al., Tet. Letts., 43: 4531-4533 (2002), (5 g, 16 mmol) in THF (50 mL) was added carefully, in portions, lithium aluminium hydride (0.9 g, 23 mmol). The reaction mixture was refluxed for 4 hours, cooled to room temperature, quenched with water and extracted with EtOAc. The aqueous phase was acidified with 2N HCl and extracted with EtOAc. The combined extracts were washed with satd sodium bicarbonate, satd. NaCl, dried with Na2SO4, filtered and evaporated to give the title compound (4.5 g, 90% yield). Mp 69-72° C. 1H NMR (300 MHz, CDCl3) δ 6.09 (s, 2H), 3.75-3.90 (m, 13H), 3.11 (t, J=5.1 Hz, 1H), 1.91 (t, J=5.8 Hz, 2H), 1.38 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 160.4, 158.8, 106.1, 90.6, 60.8, 55.8, 55.2,...
example 3
2-(4-(2-Methyl-2-(nitrosothio)propyl)piperazinyl)-2-oxoethyl 2-((2E)-3-(3,4-dimethoxyphenyl)prop-2-enoylamino)benzoate
[0477]
3a. 2,2-Dimethylthiirane
[0478] A mixture of 2,2-dimethyloxirane (25 g, 346 mmol), water (50 ml), and potassium thiocyanate (67 g, 692 mmol) was stirred at room temperature for 20 hours. The organic phase was removed, dried over Na2SO4, and filtered to give title compound (26.4 g, 87% yield). 1H NMR (300 MHz, CDCl3) δ 2.41(s, 2H), 1.62 (s, 6H).
3b. 2-Methyl-1-piperazinylpropane-2-thiol
[0479] A mixture of piperazine (44.7 g, 0.52 mol) and the product of Example 3a (15.2 g, 0.17 mmol) in toluene (70 mL) was heated at 80° C. for 6 hours. The reaction mixture was cooled, poured into water and extracted with CH2Cl2. The combined extracts were dried over Na2SO4, filtered and the solvent evaporated to give the title compound (30.5 g, 100% yield). 1H NMR (300 MHz, CDCl3) δ 2.80-2.90 (m, 4H), 2.50-2.60 (m, 4H), 2.35 (s, 2H), 1.52 (br s, 1H), 1.29 (s, 6H).
3c. 2-(4-(2-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Therapeutic | aaaaa | aaaaa |
| Pharmaceutically acceptable | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com