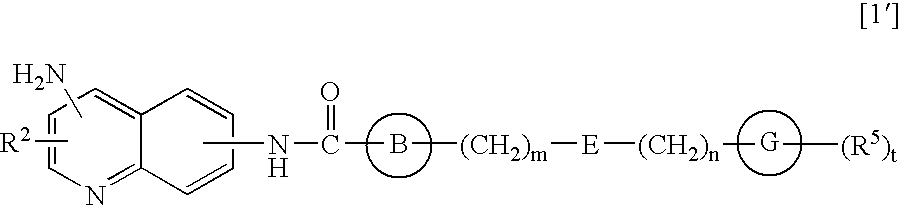
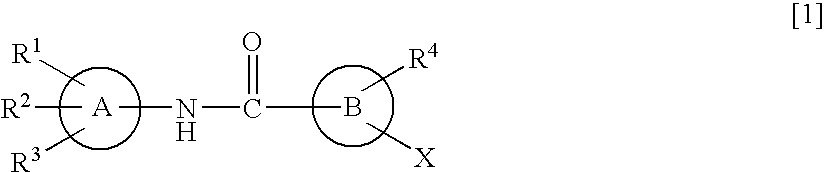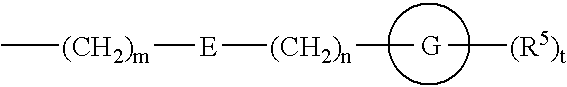Amide derivatives and nociceptin antagonists
a technology of nociceptin and amide derivatives, applied in the field of nociceptin antagonists, can solve the problems of many unknown parts in the central action mechanism, considered ineffective for visceralgia, etc., and achieve the effect of free of marked side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 1-1
Synthesis of 4,6-diamino-2-methylquinoline
[0259] The synthesis was carried out according to a reference publication (Journal of the American Chemical Society, 70, 4065, 1948).
Step 1
[0260] 4-Aminoacetoanilide (150.2 g, 1 mol) was added to a solution of methyl acetoacetate (136.8 g, 1.1 mol) in methanol (450 ml) and the mixture was refluxed under heating for 17 hours. The reaction vessel was cooled to 0° C. and the resulting white precipitate was collected by filtration to give methyl β-(p-acetamidophenylamino)crotonate (231.5 g, 93%, white crystals).
Step 2
[0261] Methyl β-(p-acetamidophenylamino)crotonate (231.5 g, 0.93 mol) obtained in Preparation Example 1-1, Step 1 was added by small portions to Dowtherm A (trademark, 600 ml) which underwent refluxing under heating. The mixture was refluxed under heating for 10 minutes and the reaction mixture was cooled to room temperature. The resulting precipitate was collected by filtration and washed with ethyl acetate. The obtained cru...
preparation example 1-2
Synthesis of 4,6-diamino-2-methylquinoline
Step 1
[0264] To 5-nitroisatin (19.21 g, 0.1 mol) were added acetone (36.7 ml, 0.5 mol) and aqueous ammonia (100 ml) and the mixture was heated in an autoclave at 100° C. for 12 hr. The reaction mixture was cooled to room temperature and the resulting crystals were collected by filtration and washed with water. The obtained crystals were dried by heating under reduced pressure to give 2-methyl-6-nitroquinoline-4-carboxamide (18.30 g, 79%).
Step 2
[0265] To 2-methyl-6-nitroquinoline-4-carboxamide (231 mg, 1 mmol) obtained in Preparation Example 1-2, Step 1 was added an aqueous sodium hypochlorite solution (0.851 ml,1.2 mmol) and the mixture was stirred at 0° C. for 2.5 hr. The reaction mixture was added dropwise to hot water (10 ml) under reflux with heating and the mixture was refluxed under heating for 20 min. The reaction mixture was cooled to room temperature and the resulting crystals were collected by filtration and dried by heating ...
preparation example 1-3
Synthesis of 6-amino-2-methyl-4-methylaminoquinoline
Step 1
[0267] N-(4-Hydroxy-2-methyl-6-quinolyl)acetamide (4.32 g, 20 mmol) and phosphorus oxychloride (9.32 ml, 100 mmol) were heated at 100° C. for 15 min. The reaction mixture was cooled to room temperature and poured into ice water. Thereto was added 28% aqueous ammonia to make the solution alkaline. The resulting insoluble matter was collected by filtration, washed with ether and water, dried under reduced pressure at 80° C. to give N-(4-chloro-2-methyl-6-quinolyl)acetamide (6.85 g, crude, yellow solid).
Step 2
[0268] A suspension of N-(4-chloro-2-methyl-6-quinolyl)acetamide (4.0 g, crude) obtained in Preparation Example 1-3, Step 1 and 85% potassium hydroxide (6.6 g, 100 mmol) in N-methylformamide (100 ml) was heated at 170° C. for 3 hr 20 min. The reaction mixture was cooled to room temperature and the reaction mixture was diluted with chloroform and water. The chloroform layer was washed successively with saturated aqueou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com