Polypeptide delivery system
a delivery system and polypeptide technology, applied in the field of expression constructs, can solve the problems of destroying implanted cells, restricting industry-wide adoption, and reducing and achieve the effect of improving the gene delivery system and increasing the secretion of heterologous gene products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cloning of the ISS-pST Construct
[0039] The pST gene was obtained from Southern Cross Biotechnology Pty Ltd in an E. coli bacterium. The plasmid containing the pST gene, pMG939, was isolated from the bacterium using standard plasmid preparation techniques. The PCR primers were designed to amplify the pST gene, add an Xho I site to the 5′ end and an Xba I site to the 3′ end to enable ligation events.
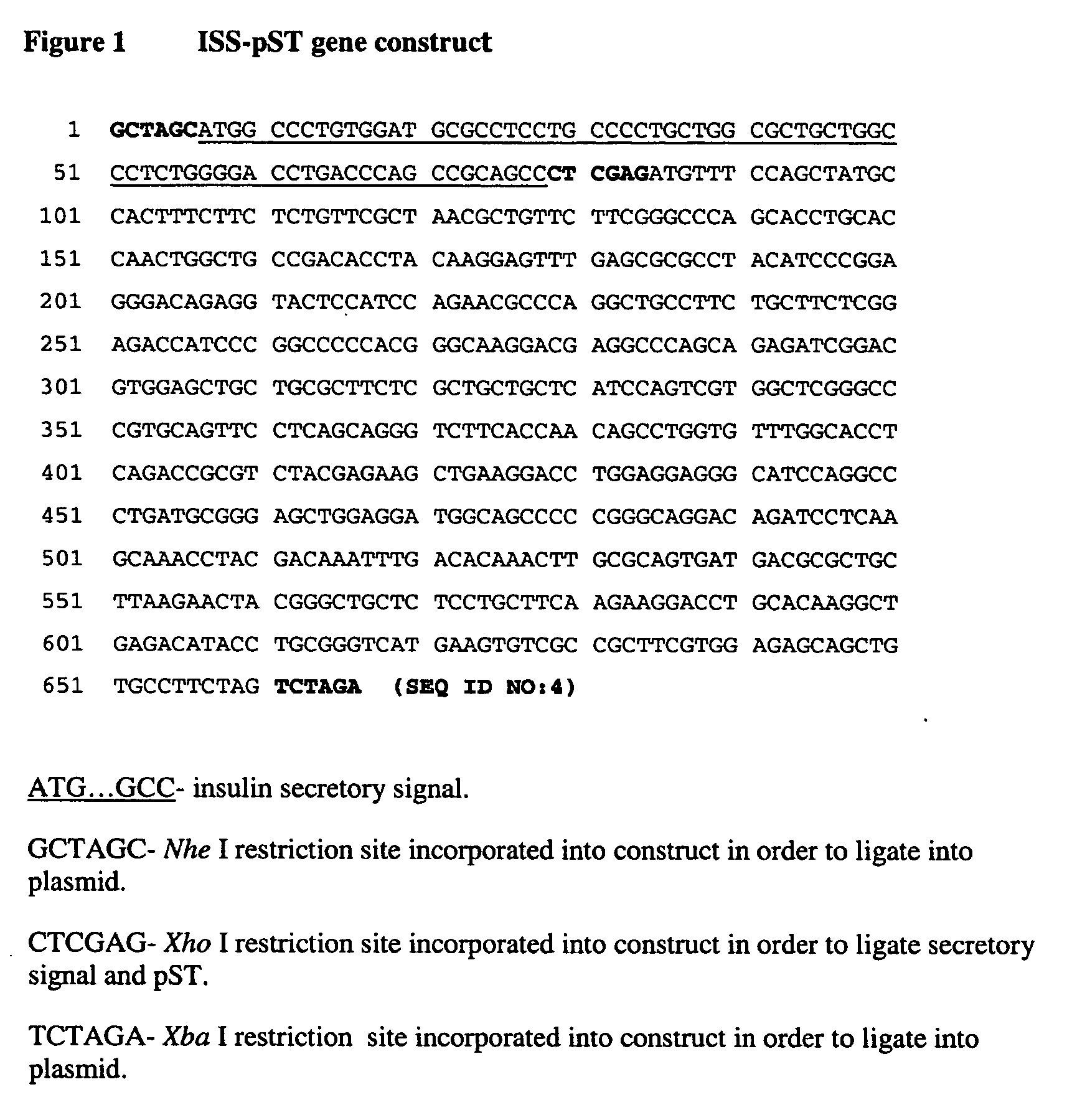
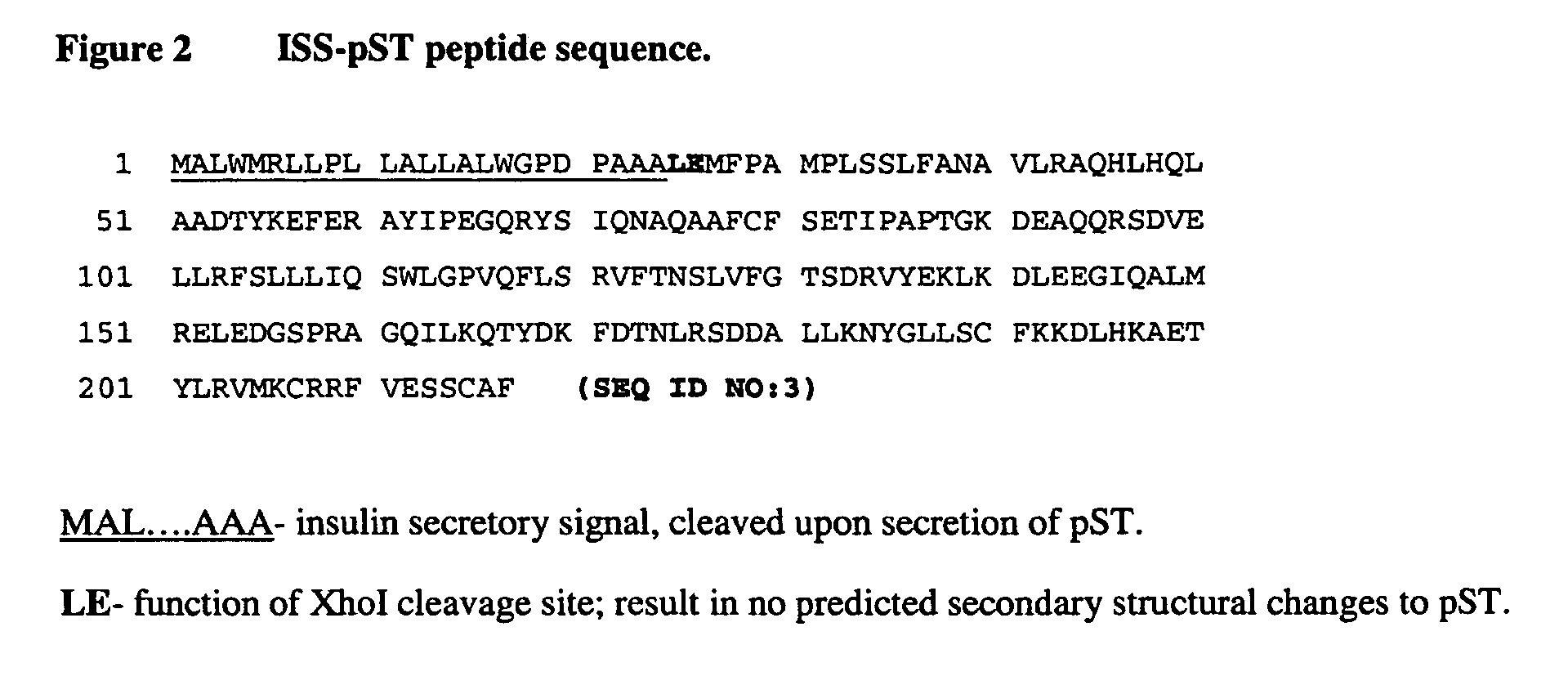
[0040] The modified pST gene sequence was subsequently ligated to a secretory signal sequence (ISS) derived from the preproinsulin cDNA. Nhe I (GCTAGC) and Xba I (TCTAGA) restriction sites were constructed in front of the ISS start codon and after the 3′ terminal codon of pST, respectively, to allow incorporation into the pCI-neo plasmid (Promega). The pST fusion construct was subsequently isolated and sequenced to verify the coding region (FIG. 1).
[0041] Transfection of rat myoblast (L6) cells (pST gene incorporation into cells) was performed, with LipoTAXI (Stratagene), 2 hrs after th...
example 2
Preparation of the Porcine Somatotropin-Rat Myoblast (L6) Immunoneutral Expression System (pST-L6IXS)
[0043] The encapsulation procedure described in Basic et al, 1996, was followed with the following modifications.
[0044] Encapsulation of cells at room temperature, utilises calcium chloride (or lactate) [100 mM] to gel the alginate [1.5% w / v] droplets followed immediately by washing with saline (0.9% NaCl) then resuspending in poly-L-lysine [0.05%] for 5 min. Calcium chloride crosslinking for 10 min at 37° C. resulted in an alginate matrix that was more compatible with cell viablity.
[0045] After the poly-L-lysine coating and saline washes another alginate layer is added. Sodium citrate [55 mM] treatment for 4 min at room temperature softens the capsule to a consistency that increases the difficulty of further manipulation. Cell viablity is apparently reduced to 98%.
[0046] Procedural and equipment modifications to the encapsulation protocol improved the efficiency (time and resour...
example 3
Pilot Experiment (1) Involving Implantation of pST-L6IXS in Pigs
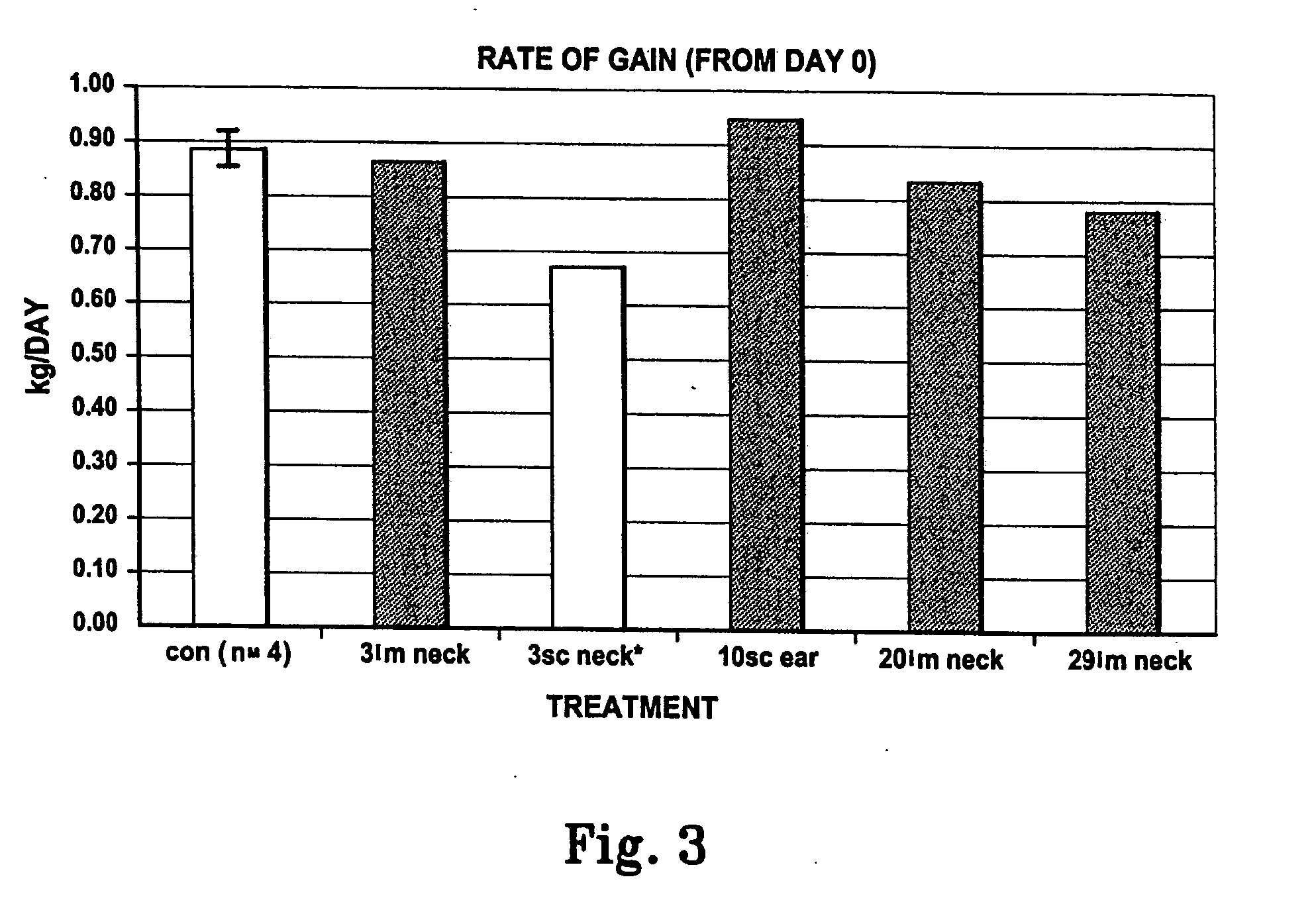
[0047] Preliminary results obtained with the pST-L6IXS, administered to growing mice, indicate enhanced growth characteristics. In a pilot experiment with male pigs (n=9, mean live weight 61 kg) varying numbers of pST-L6IXS were administered in different sites (3 capsules, i.m. in the neck muscle, 3 capsules s.c. in the neck, 10 capsules s.c. at the base of the ear, 20 capsules i.m. in the neck or 29 capsules i.m. in the neck of individual animals on day 0). Blood samples (10 ml) were collected via jugular venipuncture and P2 ultra-sound (us) measurements were recorded at −14, 0, 7, 14, 21, 28 and 36 days post administration. The sites of pST-L6IXS administration were monitored for tissue reaction events throughout the experiment. On day 36 animals were euthanased and carcass analysis (back fat depth, BF(mm); eye muscle area, EMA(cm); forearm bone length, BONE(cm); heart weight, HEART(gm); spleen weight, SPLEEN(gm) and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| mean live weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com