Method of drug delivery to interstitial regions of the myocardium
a drug delivery and interstitial region technology, applied in the direction of drugs, catheters, drugs, etc., can solve the problems of not recognizing the potential of such delivery systems in treating disease substrates, difficult local delivery of easily transported molecules, and inability to deliver therapeutic agents to the interstitial space, etc., to achieve the effect of easy entry into the cardiac capillary system, rapid transportation away, and small siz
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
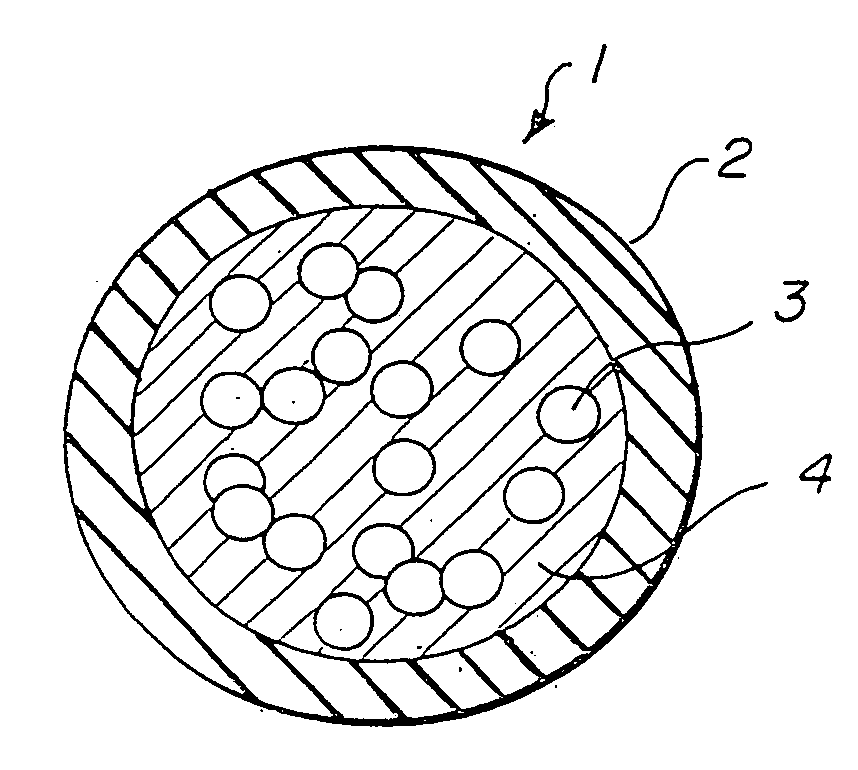
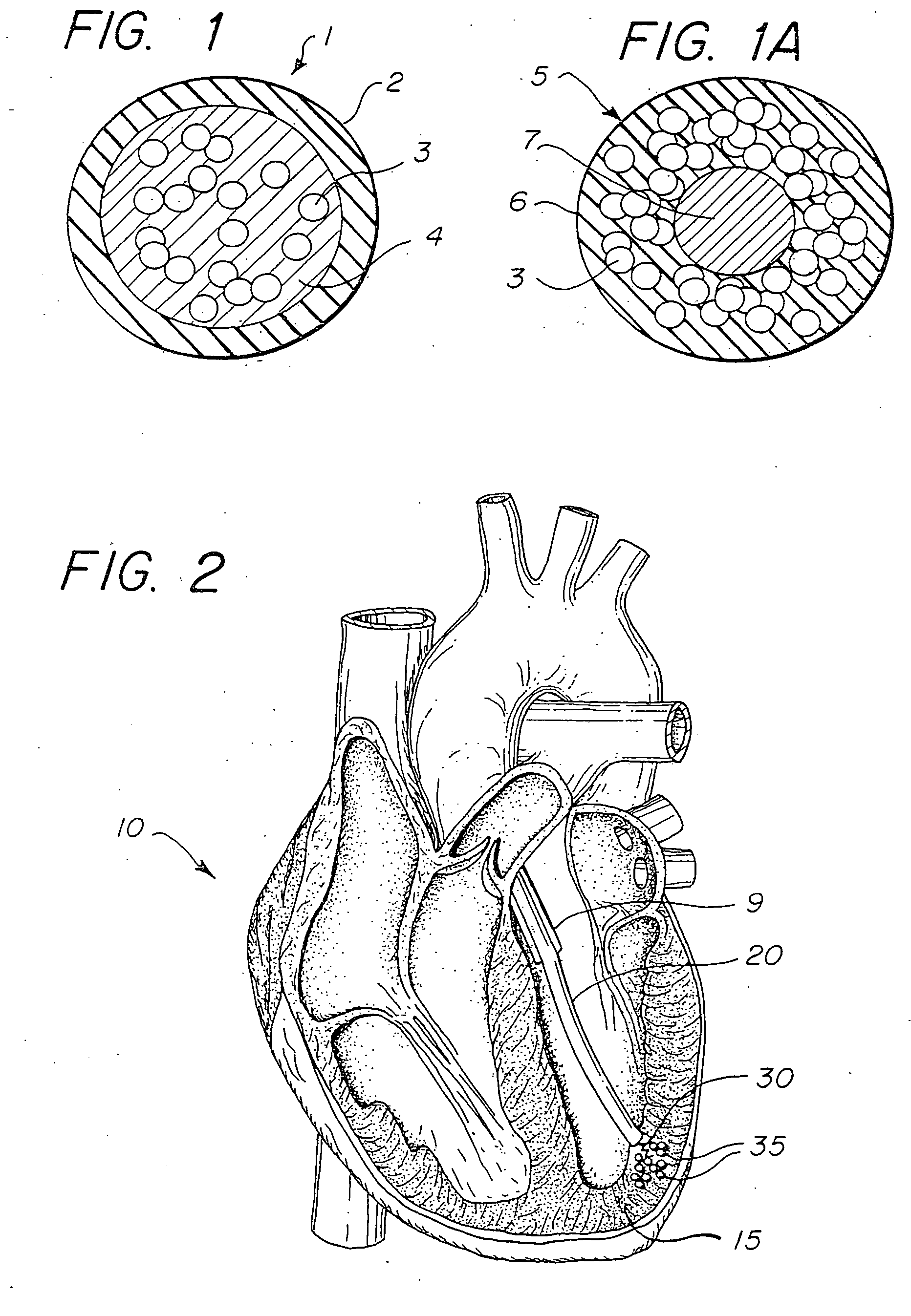
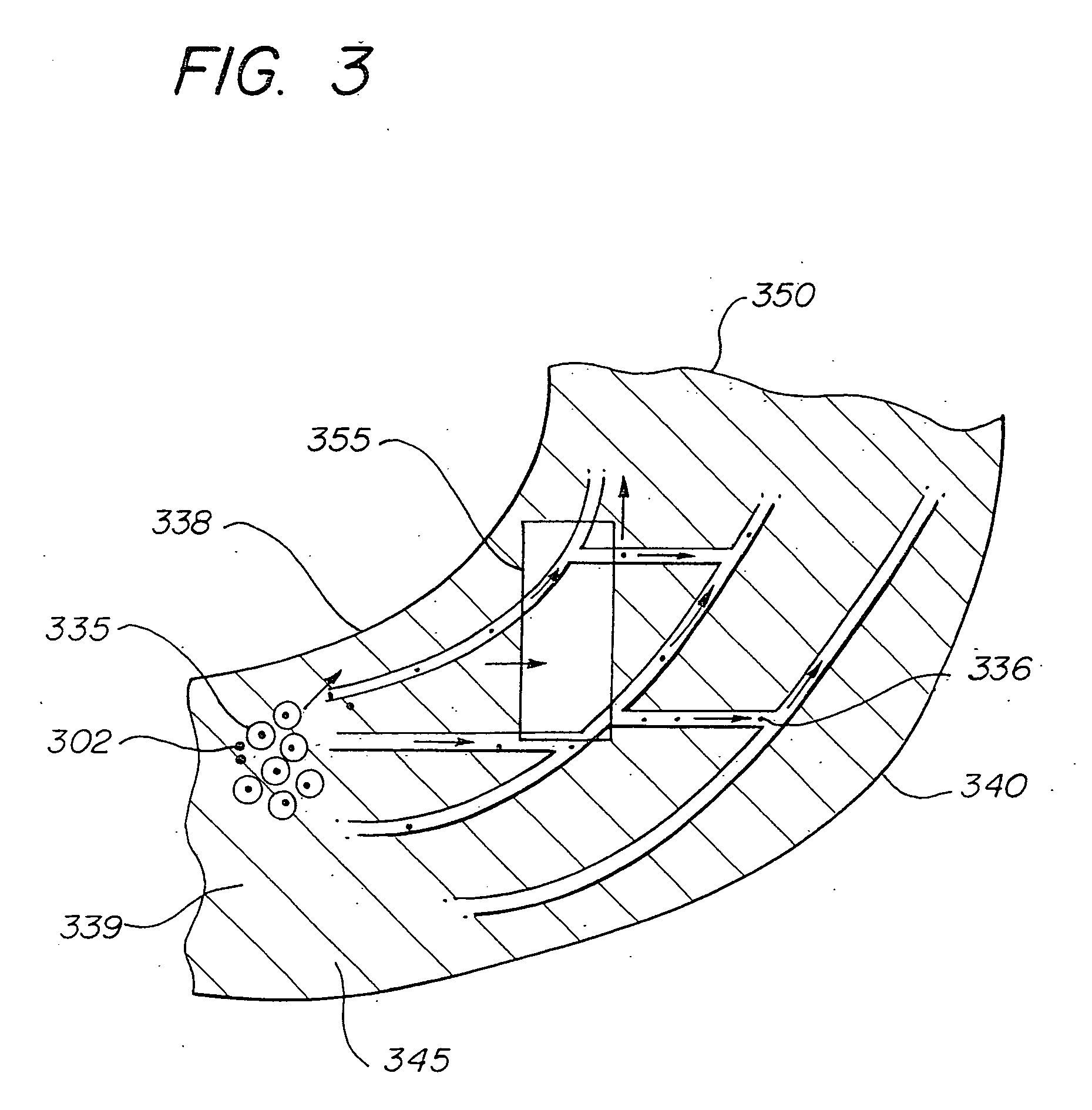
[0014]FIG. 1 illustrates a microdrug delivery system which is comprised of a compound or substance for use in delivering a therapeutic agent to the heart. The compound is comprised of numerous capsules 1 which are made up of an encapsulating layer 2 which may form a microsphere formulated from Prolease™ or other biodegradable microsphere material, or from vesicle forming lipids which may form a liposome or micelle, and a therapeutic agent 3 within the encapsulating layer. Therapeutic agent may be embedded in a biodegradable polymer, or in a carrier fluid 4. The encapsulating layer is typically pharmacologically inactive, although techniques to make it active to promote cellular uptake and / or receptor binding are known in the art. The therapeutic agent may be any of a wide variety of drugs and other compounds used for treatment of various ailments of the heart. The capsules are carried within a solution such as pH controlled saline to create a slurry which can be injected into the he...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| radius | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com