Method for halogenating or radiohalogenating a chemical compound
a chemical compound and radiohalogenation technology, applied in the field of labeling a chemical compound, can solve the problems of many difficulties in synthesizing desired radiohalogenated compounds, limited availability of suitable organic starting materials for radiolabeling reaction, and inability to work well on small scales
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Organotrifluoroborates
[0035] The procedure for obtaining organotrifluoroborate salts is shown schematically in Scheme 2.
[0036] A solution of a starting organic compound, such as phenylacetylene, (1.02 g, 10.0 mmol) in 20 mL of dry THF was cooled to −78° C. under argon. n-Butyllithium (6.25 mL, 1.60 M in hexane, 10 mmol) was added dropwise and the solution was stirred for one hour. Trimethylborate (1.58 g, 15.0 mmol, 1.5 equiv) was then added dropwise at −78° C. and the solution stirred for one hour. The reaction mixture was then allowed to warm to −20° C. and stirred for an additional hour. A saturated aqueous solution of potassium hydrogen difluoride (4.70 g, 60.0 mmol, 6 equivalents) was added to the vigorously stirred solution. The resulting mixture was allowed to stir for one hour at −20° C., after which it was allowed to warm to room temperature. The solvent was removed under reduced pressure and the resulting white solid was dried under high vacuum for two hou...
example 2
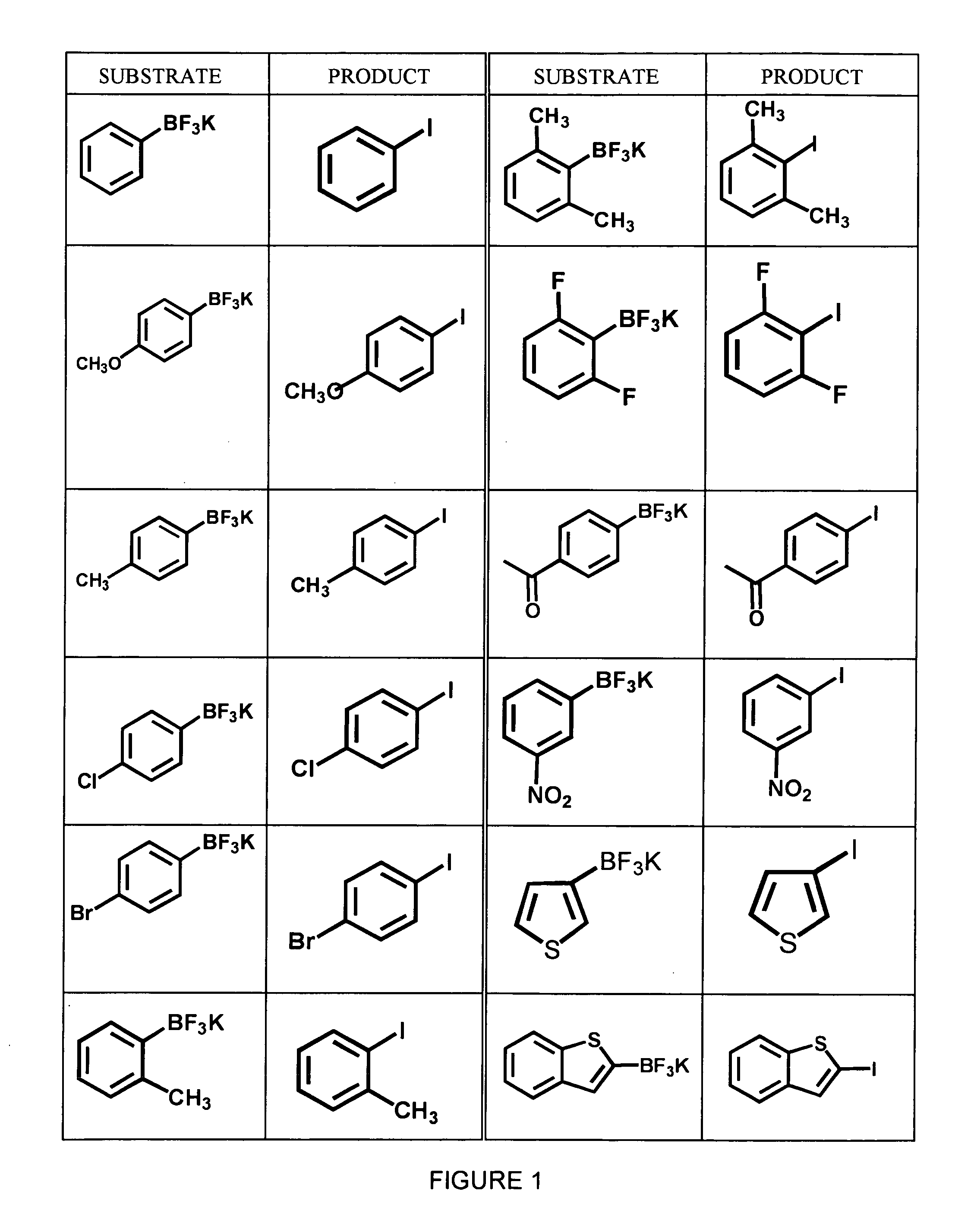
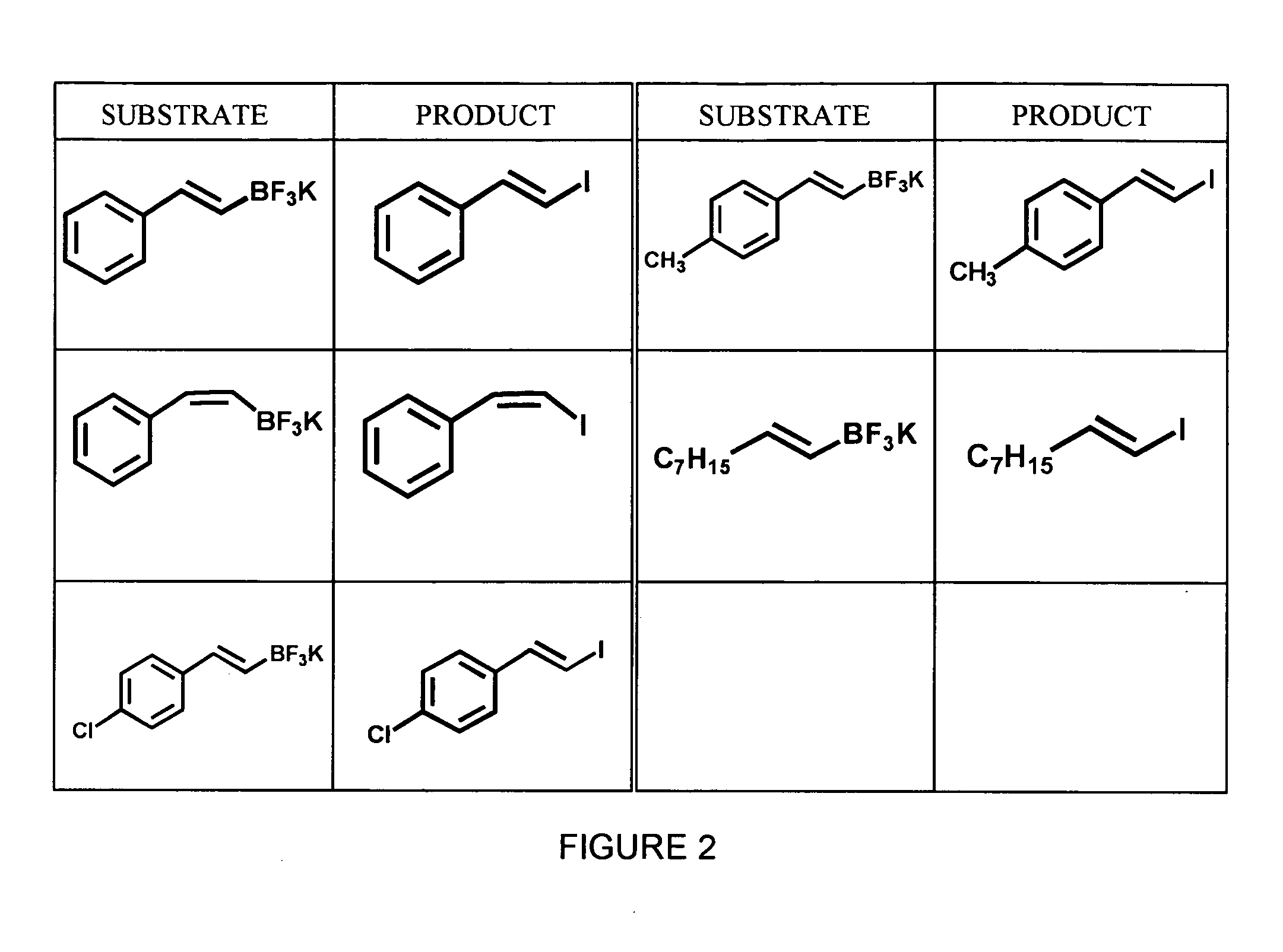
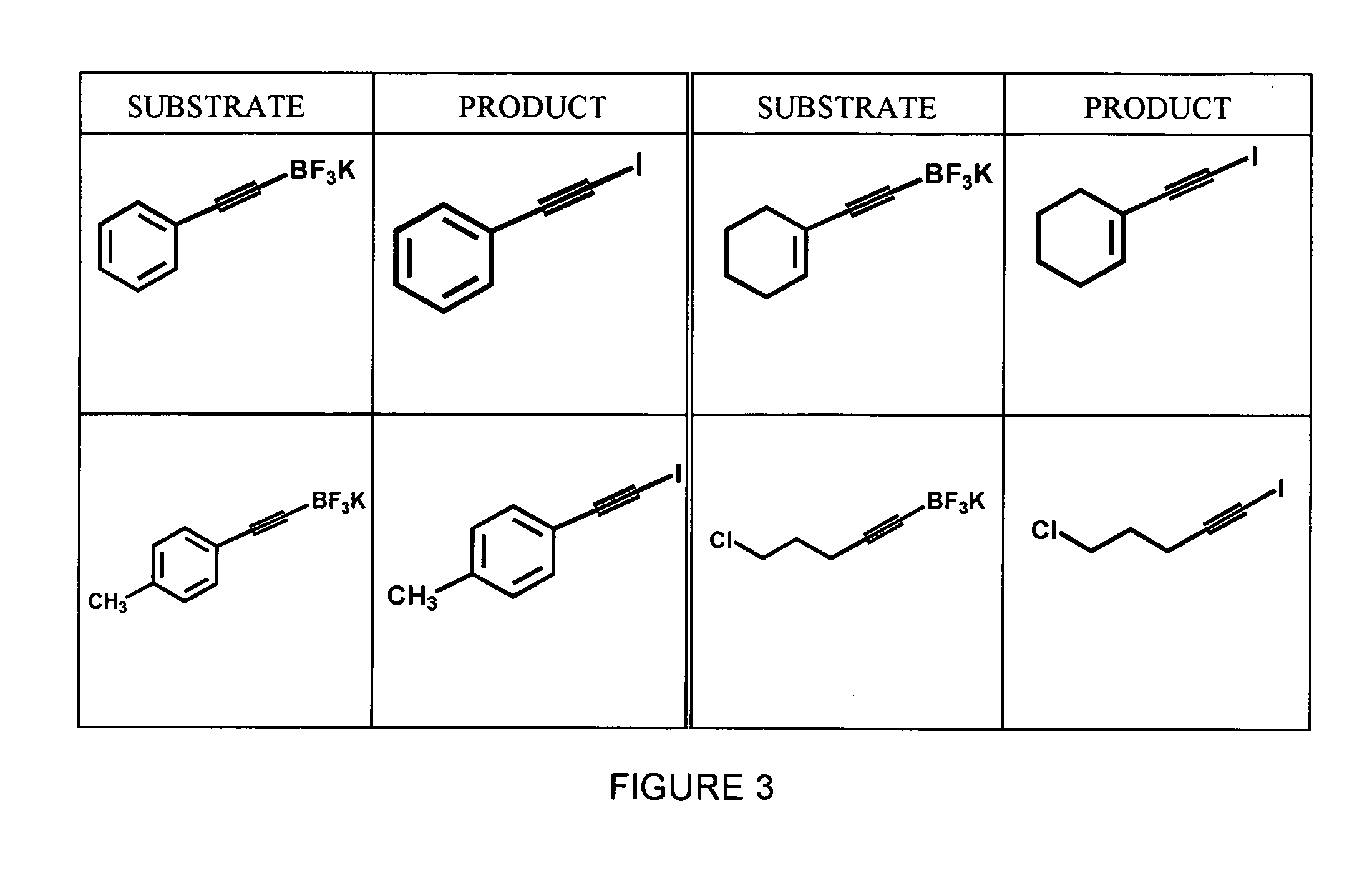
Synthesis of Aryl Iodides
[0038] A variety of aryltrifluoroborates, synthesized as described in Example 1, were subjected to radioiodination using no-carrier added Na123I and peracetic acid in 50% aqueous THF to yield the corresponding aryliodides. The general formula for this reaction is shown below in Scheme 3. All reactions were carried out using dry solvents under an inert atmosphere.
example 2a
Radiosynthesis of 4-[123I]iodoanisole (Representative Procedure)
[0039] Para-methoxyphenyltrifluoroborate (100 μL of 5.2×10−2 solution in 50% aqueous tetrahydrofuran) was placed in a 2 mL Wheaton vial containing no-carrier-added Na123, (37 MBq in 0.1% aqueous NaOH). To this was added peracetic acid (100 μL, 0.3% solution in methanol). The reaction vial was sealed, covered with aluminum foil, and the mixture stirred for 10 min at room temperature. A drop of 10% aqueous sodium thiosulfite was added to decompose excess iodine and the radioiodinated product was isolated by passing it through a silica gel Sep-Pak cartridge using petroleum ether:ethyl acetate (50:1) as eluent. The radiochemical purity of 4-[123I]iodoanisole was determined by radio-TLC (aluminum backed silica gel plate, hexane:ethyl acetate=50:1); Rf=0.55. The TLC retention time (and that of all products) was identical to that of authentic samples. The radiochemical purity was 98% and the radiochemical yield was 88%. The t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| radioactive | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com