Method for manufacturing nanophase TiC-based composite powders by metallothermic reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
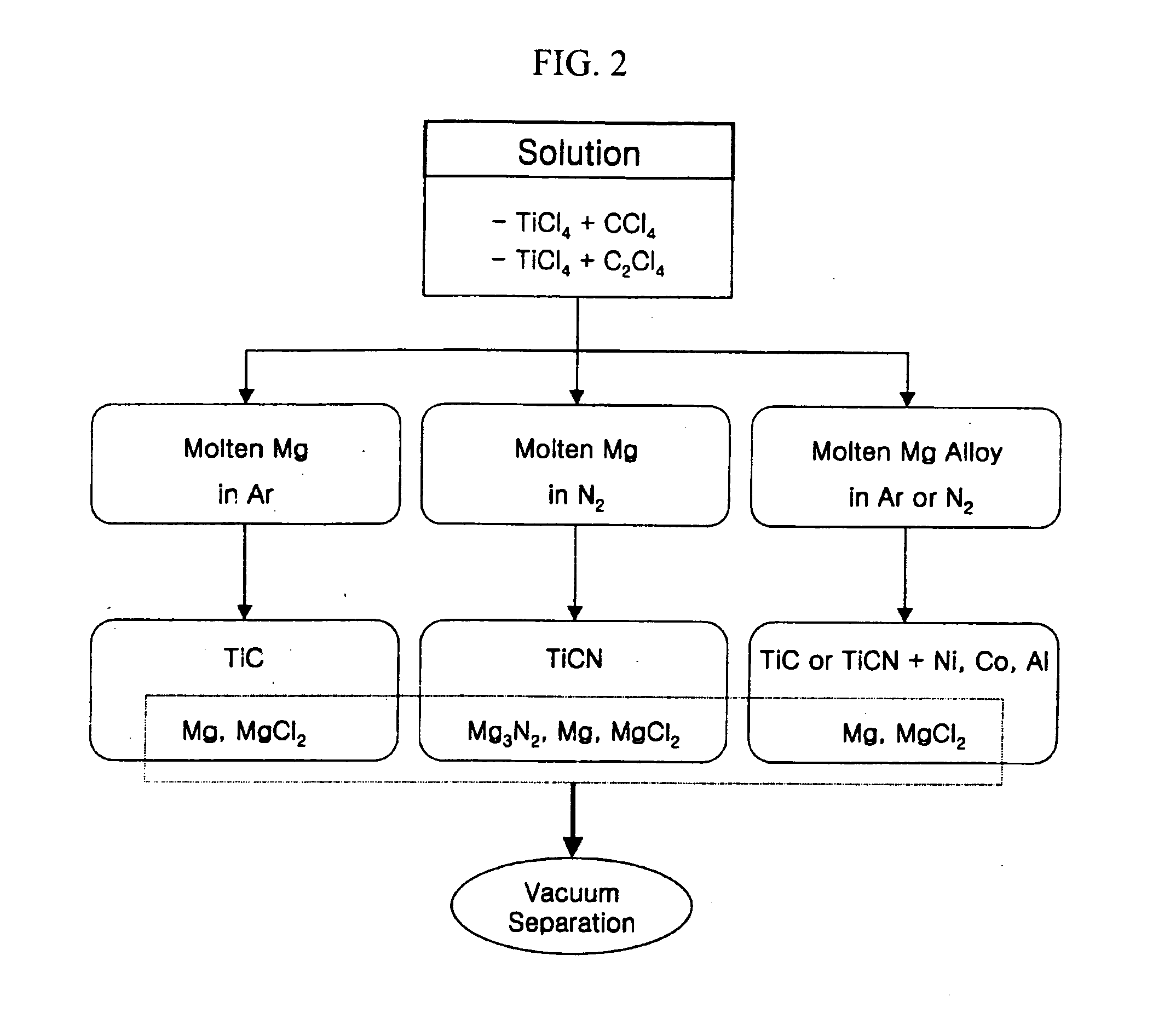
[0070] This Example describes a method for manufacturing TiC by means of metallothermic reduction. This Example was based on the following reaction 2.
TiCl4(g)+CCl4(g)+4Mg(l)→TiC(s)+4MgCl2(l) (2)
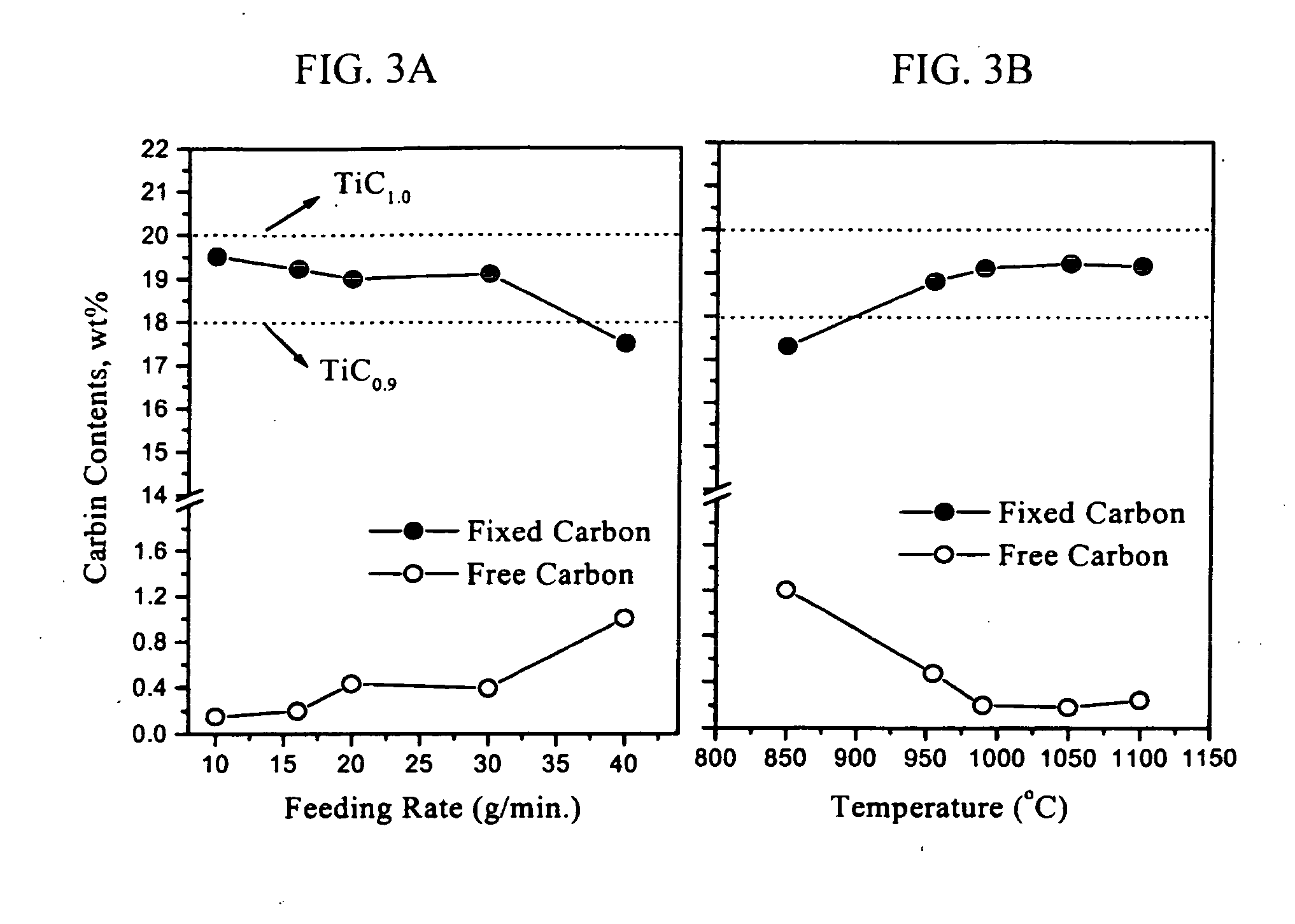
[0071] The purities of TiCl4 and CCl4 were 99.9%. First, 1 mole (189.7 g) of TiCl4 was prepared. Since the boiling point of CCl4 is lower than that of TiCl4, that is, more volatile, CCl4 is first evaporated. Accordingly, some amount of CCl4 was condensed at the inner wall of a reactor and thus did not react with molten magnesium. This lowered the content of fixed carbon in final TiC compound.
[0072] To avoid this disadvantage, 1.05 moles (157.3 g) of CCl4 was set as a minimum amount and 1.15 moles (172.3 g) of CCl4 was set as a maximum amount, relative to the stoichiometric ratio (149.8 g) with TiCl4.
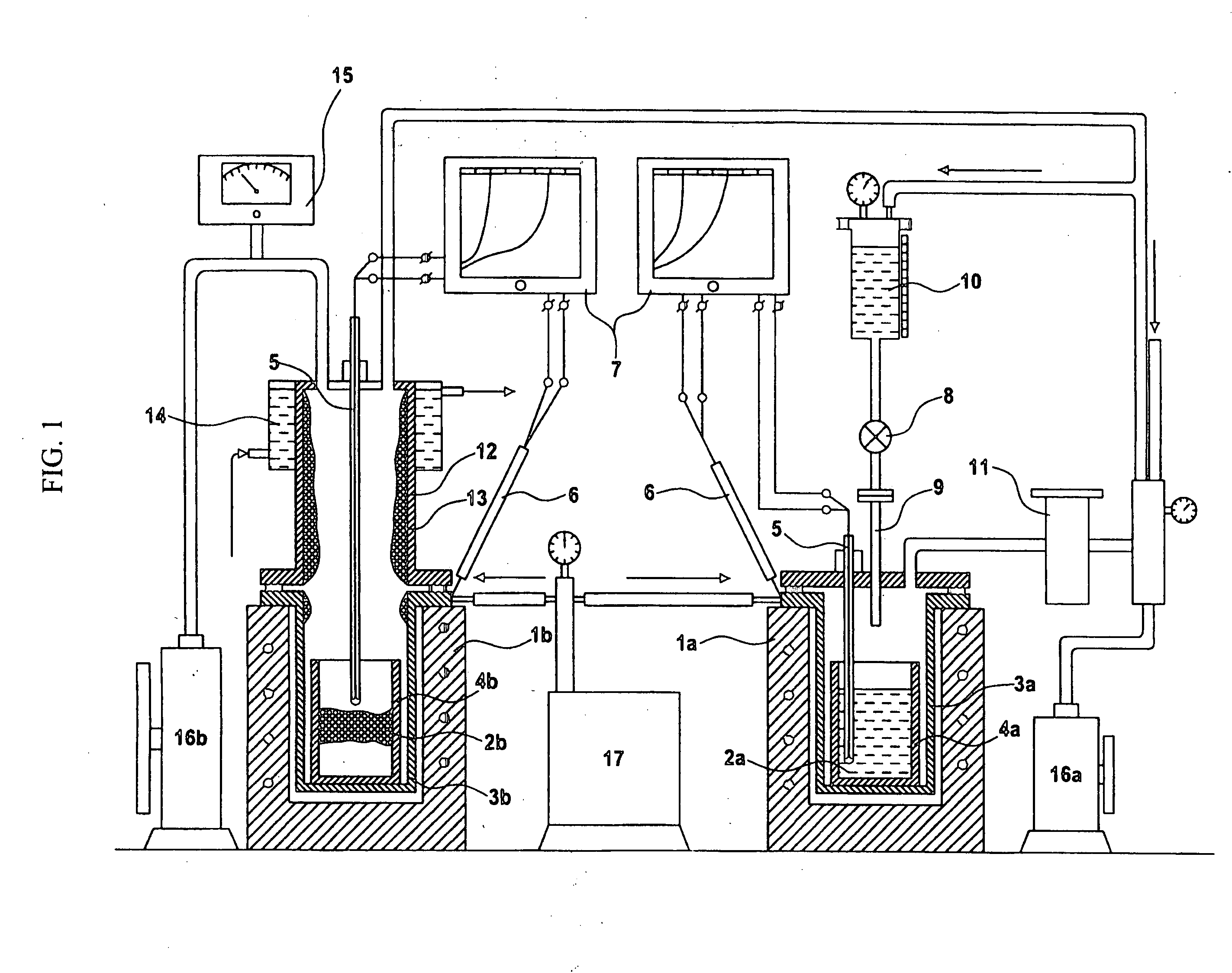
[0073] The purity of magnesium, a reducing agent of a starting solution of titanium tetrachloride in carbon tetrachloride, was 99.9%. Although 4 moles of magnesium corresponds to 97.3 g, a su...
example 2
[0089] This Example describes a method for manufacturing TiCN by means of metallothermic reduction. Specifically, a TiCN compound having a composition TiC0.5N0.5 was manufactured in the same manner as in Example 1 except that a solution of TiCl4 in C2Cl4 was used as a starting solution and nitrogen (N2) gas was used to create the atmosphere of a closed container instead of argon (Ar) gas.
[0090] The TiCN compound having a composition TiC0.5N0.5 was manufactured, based on the following reaction 4:
TiCl4(g)+1 / 4C2Cl4(g)+5 / 2Mg(l)+(1 / 4N2(g))→TiC0.5N0.5(s)+5 / 2MgCl2(l) (4)
[0091] TiCl4, C2Cl4 and Mg were used in the amounts of 189.7 g, 87.0 g and 250 g, respectively. Changes in the contents of free carbon, fixed carbon and fixed nitrogen at various feeding rates of the starting solution into molten magnesium and various reaction temperatures are shown in FIG. 6.
[0092] When Ti, separated by the reduction of Mg, was bonded to ½C atom, bonding with a nitrogen atom followed, unlike in the ma...
example 3
[0100] This Example describes a method for manufacturing a TiC+Ni (Al, Co) composite or TiCN+Ni (Al, Co) composite by means of metallothermic reduction.
[0101] Specifically, the TiC+Ni composite was manufactured in the same manner as in Examples 1 and 2 (e.g., reaction temperature: above 1000° C. and feeding rate: below 20 g / min) except that molten magnesium alloy including at least one metal selected from nickel (Ni), aluminum (Al) and cobalt (Co) was used instead of pure magnesium. In this Example, molten Mg—Ni alloy was used as a reducing agent.
[0102] The TiC+Ni composite was manufactured, based on the following reaction 5:
TiCl4(g)+1 / 2C2Cl4(g)+3Mg / (Ni)(l)→TiC(s)+(Ni)(s)+3MgCl2(l) (5)
[0103] Referring to the reaction 5, since Al or Co can be used instead of Ni, a TiC(s)+Al(s) composite or TiC(s)+Co(s) composite can be manufactured. In addition, in the case where nitrogen gas was used to create the atmosphere of the closed container, TiCN(s)+Al, Ni, Co(s) composites can be manuf...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com