Bioproduction of astaxanthin using mutant carotenoid ketolase and carotenoid hydroxylase genes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
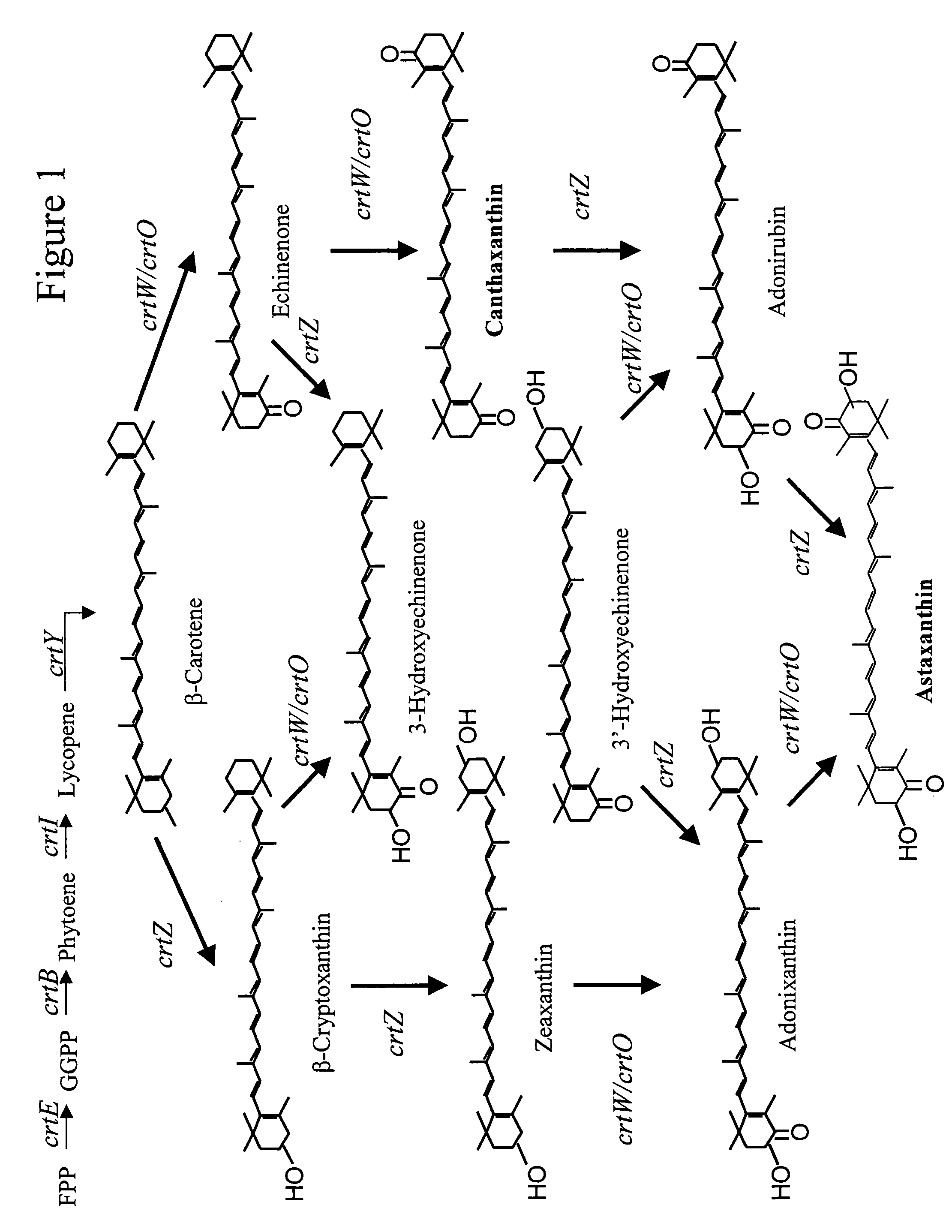
Selection of the crtOZ Plasmid for Protein Engineering Construction of two plasmids containing different crtOZ genes
[0196] The crtO gene isolated from Rhodococcus erythropolis AN12 has previously been engineered (U.S. Ser. No. 60 / 577,970) to increase the ketolase activity. One of the mutants, crtO-SHU001, produced over 90% canthaxanthin in E. coli when coexpressed with {overscore (β)}-carotene synthesis genes. This crtO was chosen to pair with crtZ for astaxanthin production. The crtO-SHU001 gene (SEQ ID NOs: 1 and 3) was PCR-amplified from pDCQ320-SHU001 plasmid DNA (U.S. Ser. No. 60 / 577,970), using forward primer crtO-SHU001-F 5′-ACTAGTAAGGAGGAATAAACCATGAGCGCA-3′ (SEQ ID NO: 3) and reverse primer crtO-SHU001-R 5′-TGTACAGCTAGCTCACGAGCGGCTCGAACGACGCAT-3′ (SEQ ID NO: 4). Underlined are restriction sites for Spe I, Nhe I and BrsG I. The ˜1.6 kb PCR product was gel purified and cloned into pTrcHis2-Topo vector, resulting in plasmid pDCQ353. The ˜1.6 kb SpeI I / BrsG I fragment of pDCQ35...
example 2
Making Mutant Libraries
Error-Prone PCR:
[0201] The plasmid pDCQ356 was used as a template for error-prone PCR. The insert containing the crtOZ genes (SEQ ID NO: 1 and SEQ ID NO: 5; respectively) can be removed from the construct using BsrG I and Spe I digestion. A random mutant library targeting the crtOZ genes was made using error-prone PCR. The following primers were used to amplify the inserts by error-prone PCR:
(SEQ ID NO: 13)334F1 5′-GCA GCG TGC AGC TCA TGC AGT TC-3′(SEQ ID NO: 14)334r1 5′-CCA GAC CGT TCA GCT GGA TAT TAC-3′
[0202] A Clontech mutagenesis kit (Clontech Laboratories, Inc., Palo Alto, Calif.) was used for performing error-prone PCR. The following condition was used for preparing error-prone PCR reaction mixture:
TABLE 1Conditions for Error-prone PCR using Clontech Mutagenesis KitVolumes (μL)PCR grade water3710x AdvanTaq Plus Buff.5MnSO4 (8 mM)3dGTP (2 mM)150x Diversify dNTP Mix1Primer mix0Template DNA1AdvanTaq Plus Polym.1
[0203] The thermal cycling reaction was...
example 3
Screening the Mutant Libraries and Identifying the Hits
[0207] The color of cells containing pDCQ356 was light yellow. The color of the cells producing astaxanthin is red-orange. The cells that make different percentages of astaxanthin show slightly different levels of pigmentation. Therefore, the mutant colonies that produce different amounts of astaxanthin can be distinguished visually. Approximately 10,000-20,000 mutant colonies from the mutant library were screened visually. Nine putative hits were streaked on Agar plates.
[0208] A follow-up confirmation assay was performed by HPLC analysis. E. coli 10G cells containing pDCQ356 and its mutant derivatives were grown in 25 ml LB with 50 μg / mL kanamycin at 30° C.; shaking for two days. Cells were harvested by centrifugation and extracted with 50% acetone and 50% methanol. HPLC analysis of the carotenoids was performed as described in Example 1. Two of the nine crtOZ mutants produced astaxanthin as shown in Table 2.
TABLE 2HPLC Con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap