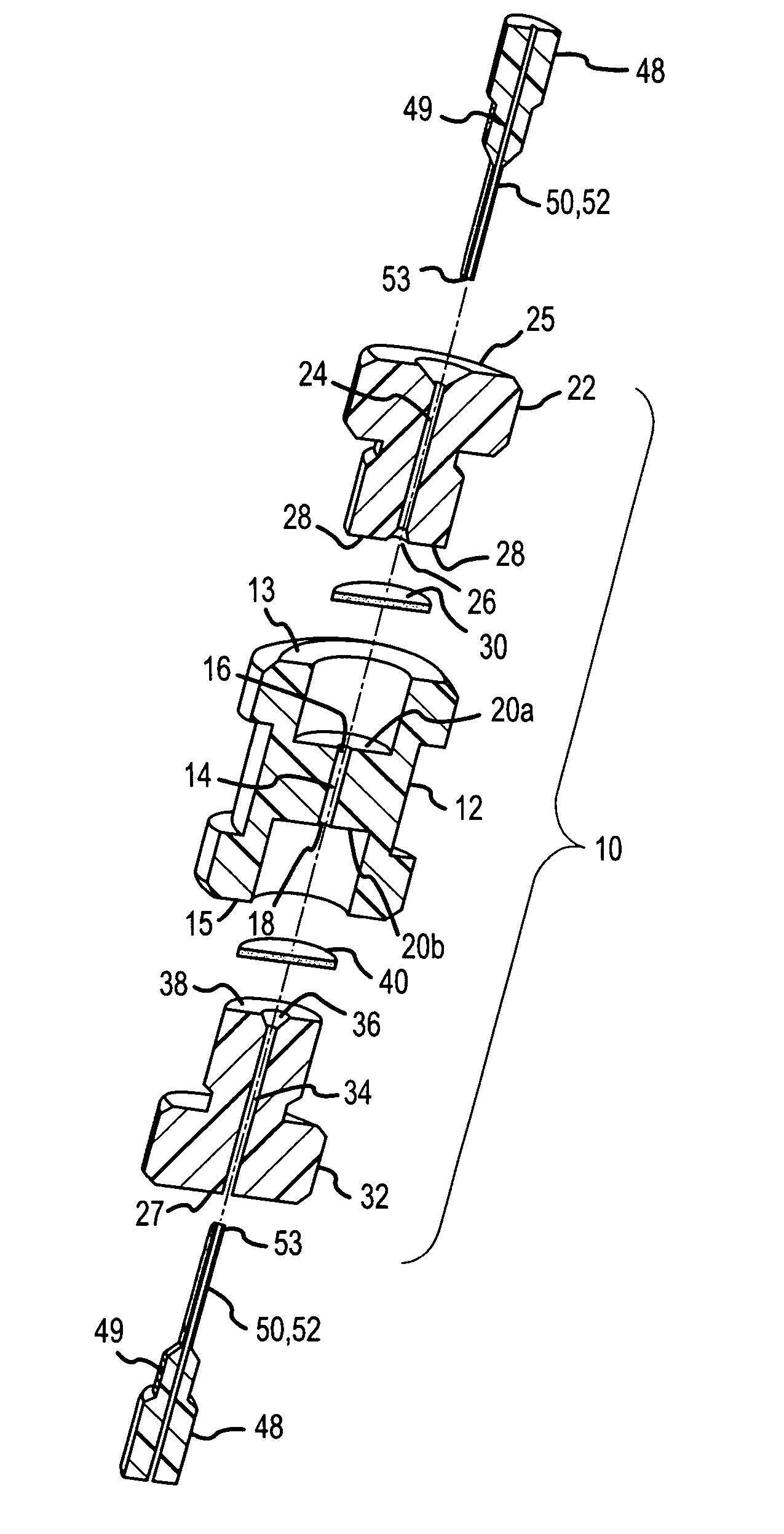
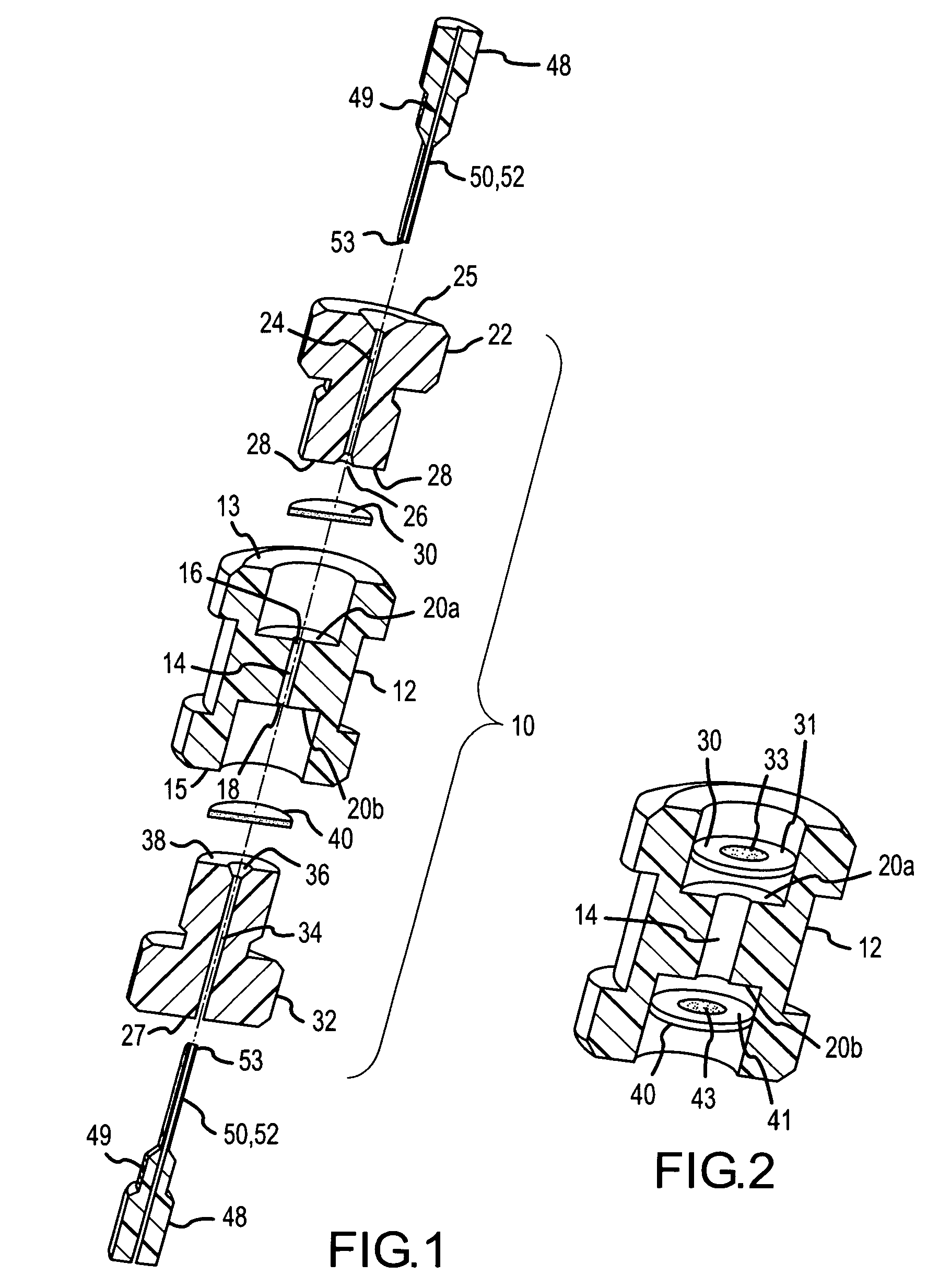
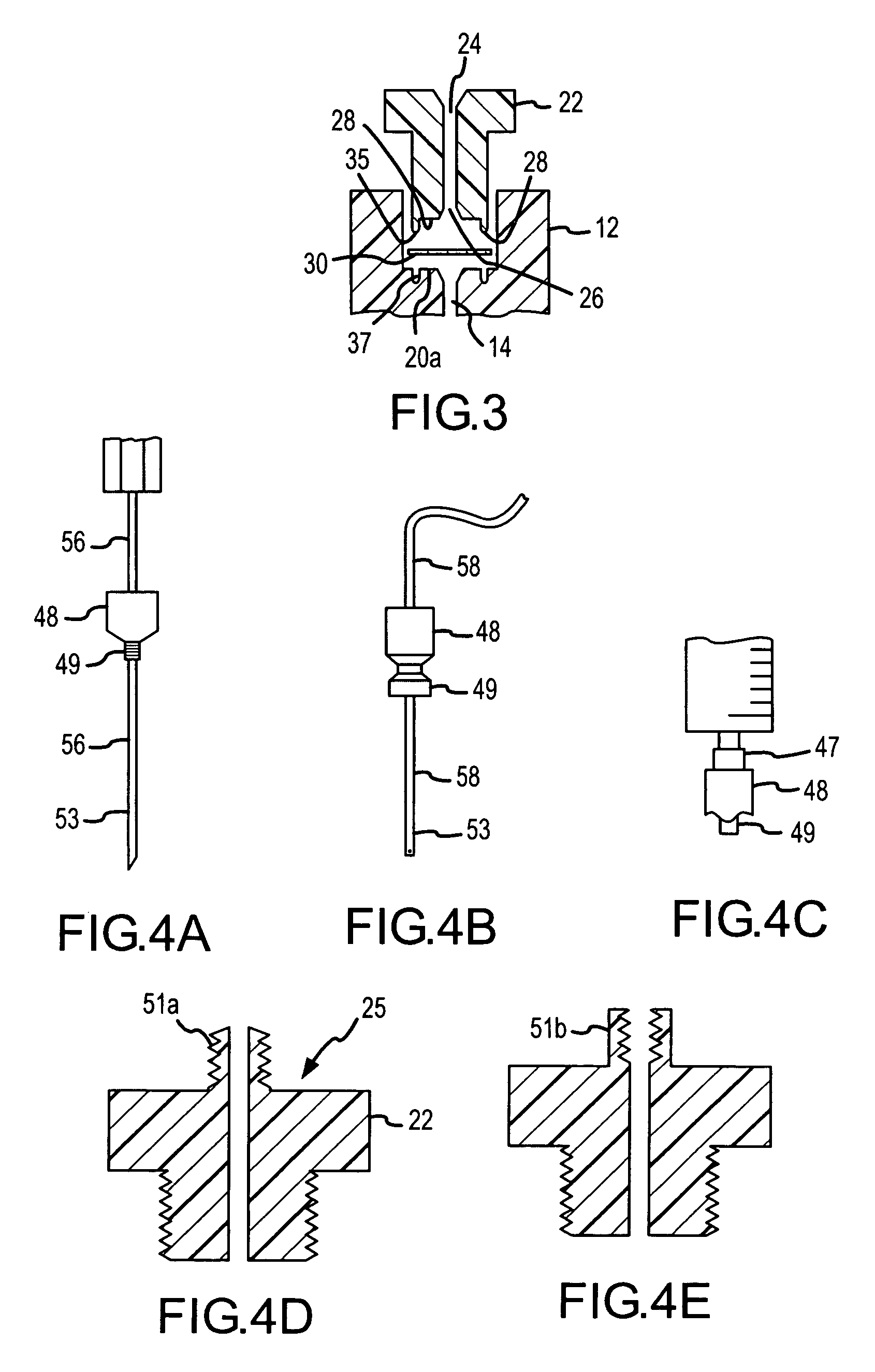
Microliter scale solid phase extraction devices
a solid phase extraction and microliter technology, applied in the field of solid phase extraction devices, can solve the problems of non-specific binding of analytes, difficult to achieve the effect of concentrating, purifying and separating analytes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Concentration and Purification of BSA with a 5 μl μSPE Cartridge
[0060]FIG. 13 illustrates an example use of the μSPE cartridge 10 to concentrate and purify a sample protein. The containment bore 14 in cartridge 10 was packed with 5 μl of anion exchange beads containing DEAE (Toyo Pearl DEAE TSK from Tosoh Biosciences, Montgomeryville, Pa.) and tested for protein concentration. The cartridge was connected on one end to a syringe filled with 1 mM bovine serum albumin (BSA) in 5 mM borate buffer and to the other end to a fused silica capillary that was mounted in a UV detection setup. As the syringe delivers through the cartridge the BSA solution at a 10 μl / min flow rate, the absorbance of the solution that exits the cartridge is recorded by the UV detector. The breakthrough curve illustrates indirectly the amount of sample retained on the cartridge. As solution is passed through the cartridge part of the BSA is retained while the unretained BSA exits the cartridge and is recorded at ...
example 2
Protein Concentration Using 2 μl and 5 μl μSPE Cartridges Packed With Different DEAE or Hydrophobic Resins
[0062] 5 μl of a crude sample of BSA was injected in a microHPLC system to asses the amount of BSA present in the sample column. The lower U.V. absorbption chromatogram in FIG. 4A shows detection of a small peak of BSA at the expected position after 10 minutes of elution. 1 ml of the same crude sample was then treated by passing the entire contents through the first sample bore of a μSPE cartridge containing 2 μl of packed Toyo Pearl DEAE 650S and eluted with 200 mM NaCl into a 5 μl sample. The 5 μl sample was then subject to the same chromatographic analysis over the microHPLC reverse phase to generate the upper chromatographic trace. Comparison of the upper trace to the lower trace in FIG. 14A illustrates that the BSA was concentrated about 20 fold using the 2 μl SPE cartridge.
[0063]FIG. 14B illustrates concentration of a mixture of lactalbumin and lysozyme by treatment thou...
example 3
Reproducibility of the Same μSPE Cartridge
[0064] To test whether the μSPE cartridges provided herein are reusable and reliable over a period of time, the same procedure for concentrating and analyzing lactalbumin and lysozyme samples depicted in FIG. 14B and described in Example 2 was repeated using the same μSPE cartridge. Between uses, the μSPE cartridges were washed with 0.20 ml a washing buffer and reconditioned with 0.20 ml of the binding buffer. As illustrated in FIG. 15, the same μSPE cartridge showed reproducible recovery of about 70% of the input lactalbumin and about 60% of the input lysozyme.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com