Photoelectrochemical determination of chemical oxygen demand
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Quantification of COD Using Photocurrent
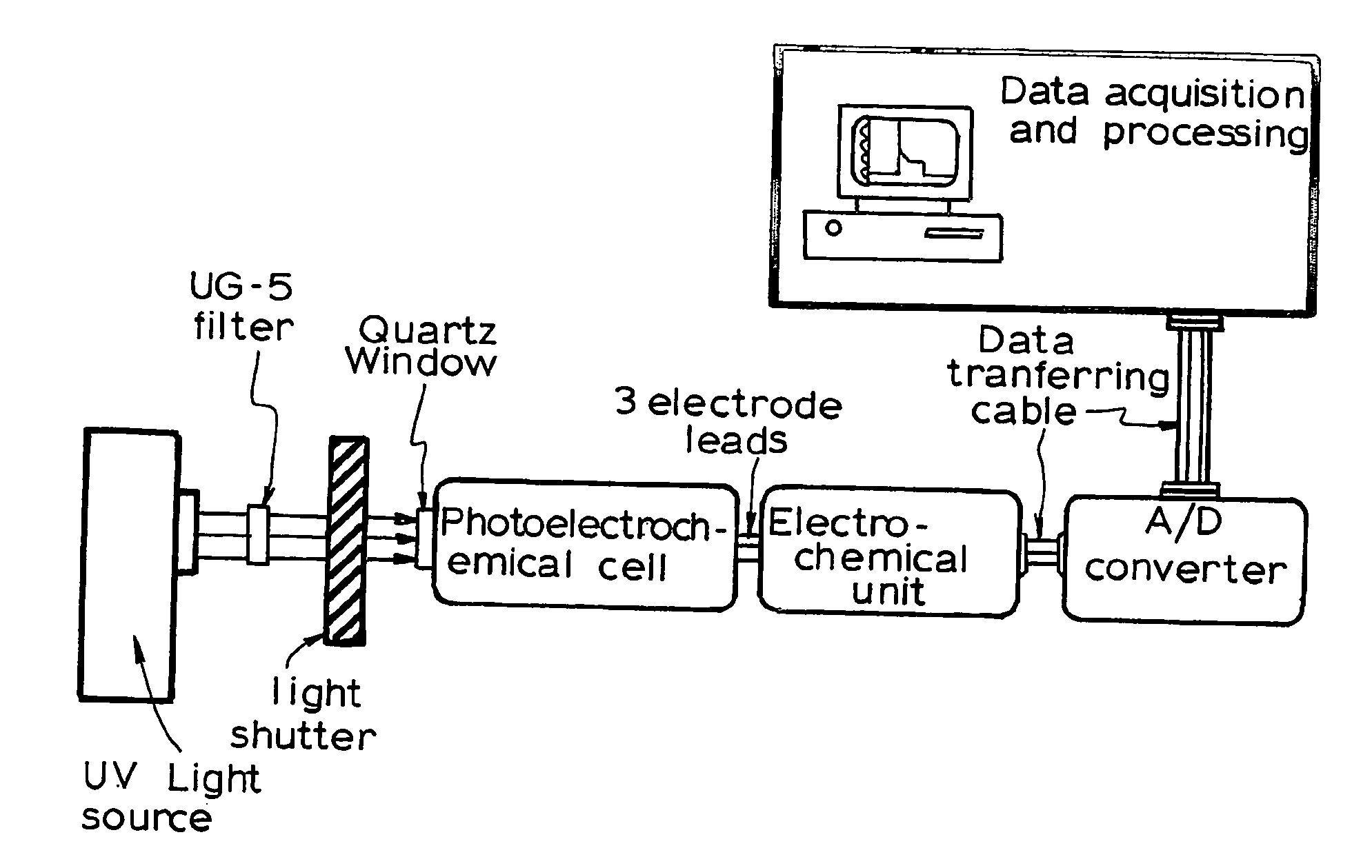
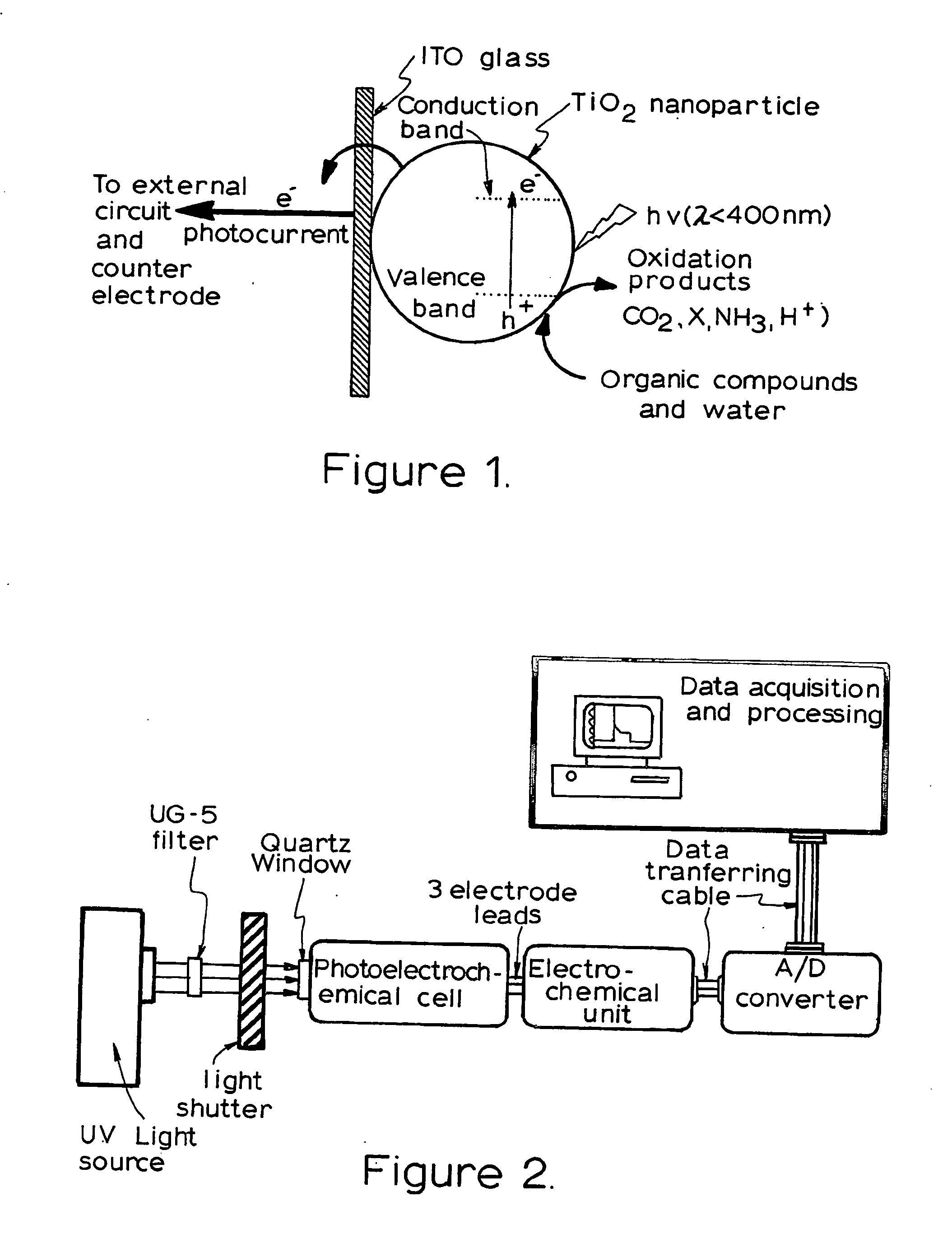
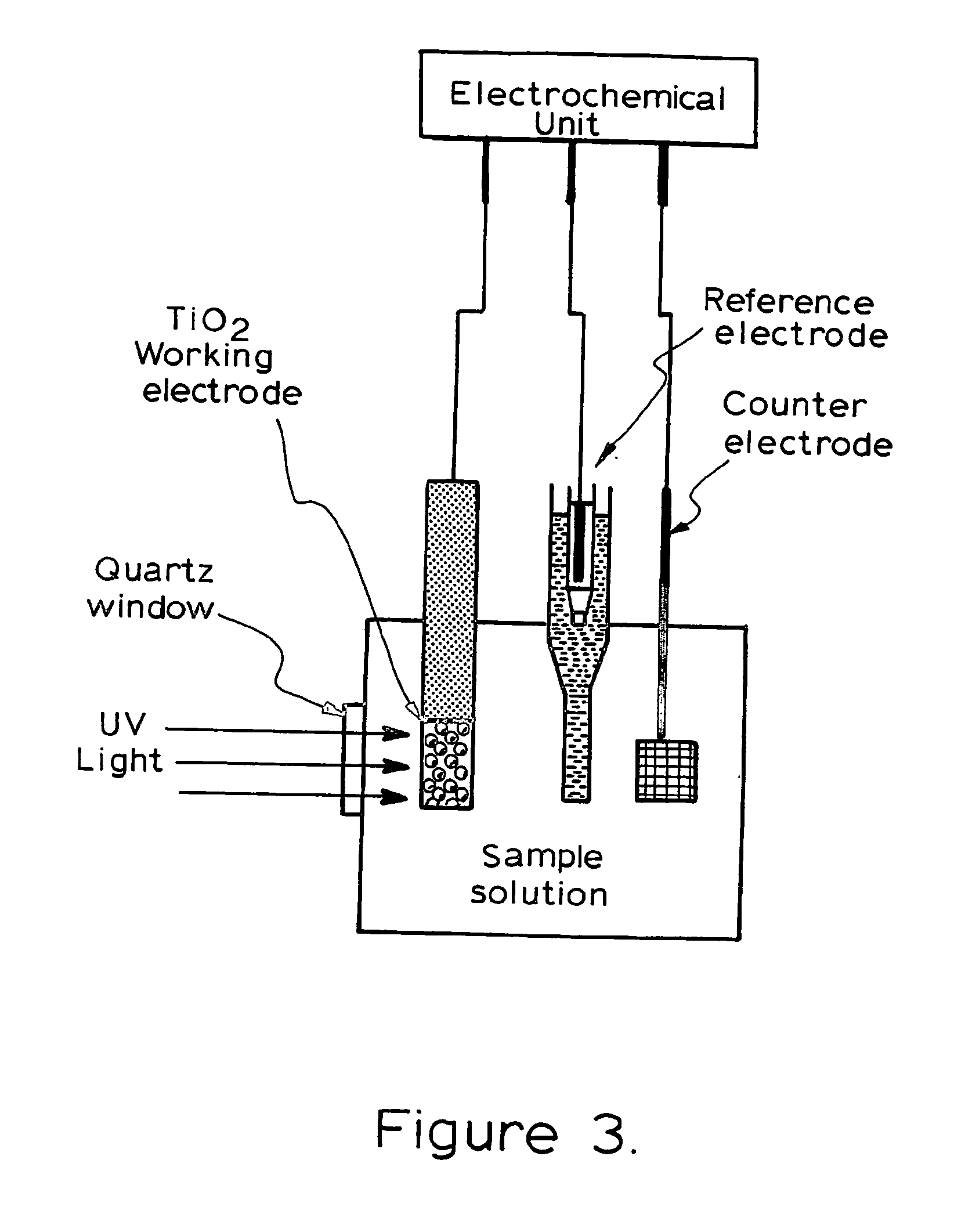
[0129] The photoelectrochemical experiment was performed in a three-electrode electrochemical batch cell with a quartz window for illumination as shown in FIG. 3 The TiO2 film electrode was placed in an electrode holder with ca. 0.65 cm2 left unsealed to be exposed to the solution for illumination and photoelectrochemical reaction. 0.1M NaNO3 solution was used as the supporting electrolyte. A potential bias of +0.2V was applied at the electrode and limiting photocurrents were obtained for different organic compound concentrations when the current reached steady state. The limiting photocurrent differences between samples and the blank 0.1M NaNO3 solution were taken as analytical signals, which are directly linear to organic compound concentrations within diffusion control. A linear relationship between the analytical signal and COD value was then acquired after the concentration was converted into COD value.
example 2
Quantification of COD Using Charges
[0130] In this case the experiment was carried out in a thin-layer photoelectrochemical cell as shown in FIGS. 4 and 5. A potential bias of +0.20V was applied and 2M NaNO3 was used as supporting electrolyte. Firstly, a 2M NaNO3 electrolyte solution was injected into the thin-layer photoelectrochemical cell with a syringe and a blank transient photoelectrolysis was run as a blank sample. The photocurrent-time profile was recorded until the photocurrent reached steady state. Then samples containing organic compounds and 2M NaNO3 were injected into the thin-layer cell and the sample transient photoelectrolysis was run. The photocurrent-time profile was recorded until the photocurrent attained steady state, indicating the organic compounds have been exhaustively photoelectrolysed. The cell was washed with supporting electrolyte solution between each sample injection. Integrating the photocurrent-time profile gives the photocatalytic oxidation charge. ...
example 3
Quantification of COD Using Charges and FIA
[0131] Besides the use of the thin-layer photoelectrochemical cell, a flow injection analysis (FIA) system was incorporated into the COD determination. With the combination of FIA, automatic COD determination was realised. In this case, the injection of samples and cell cleaning was controlled by a FIA controlling system as shown in FIG. 6 (a). Pump 1 achieves the blank sample (R1) injection and cell cleaning while Pump 2 does the sample injection (R2). A potential bias of +0.20V was applied and 2M NaNO3 was used as supporting electrolyte (blank sample). Firstly, a 2M NaNO3 electrolyte solution was pumped into the thin-layer photoelectrochemical cell by Pump 1 and a blank transient photoelectrolysis was run as a blank sample. The photocurrent-time profile was recorded until the photocurrent reached steady state. Then samples containing organic compounds and 2M NaNO3 were pumped into the thin-layer cell by Pump 2 and the sample transient ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com