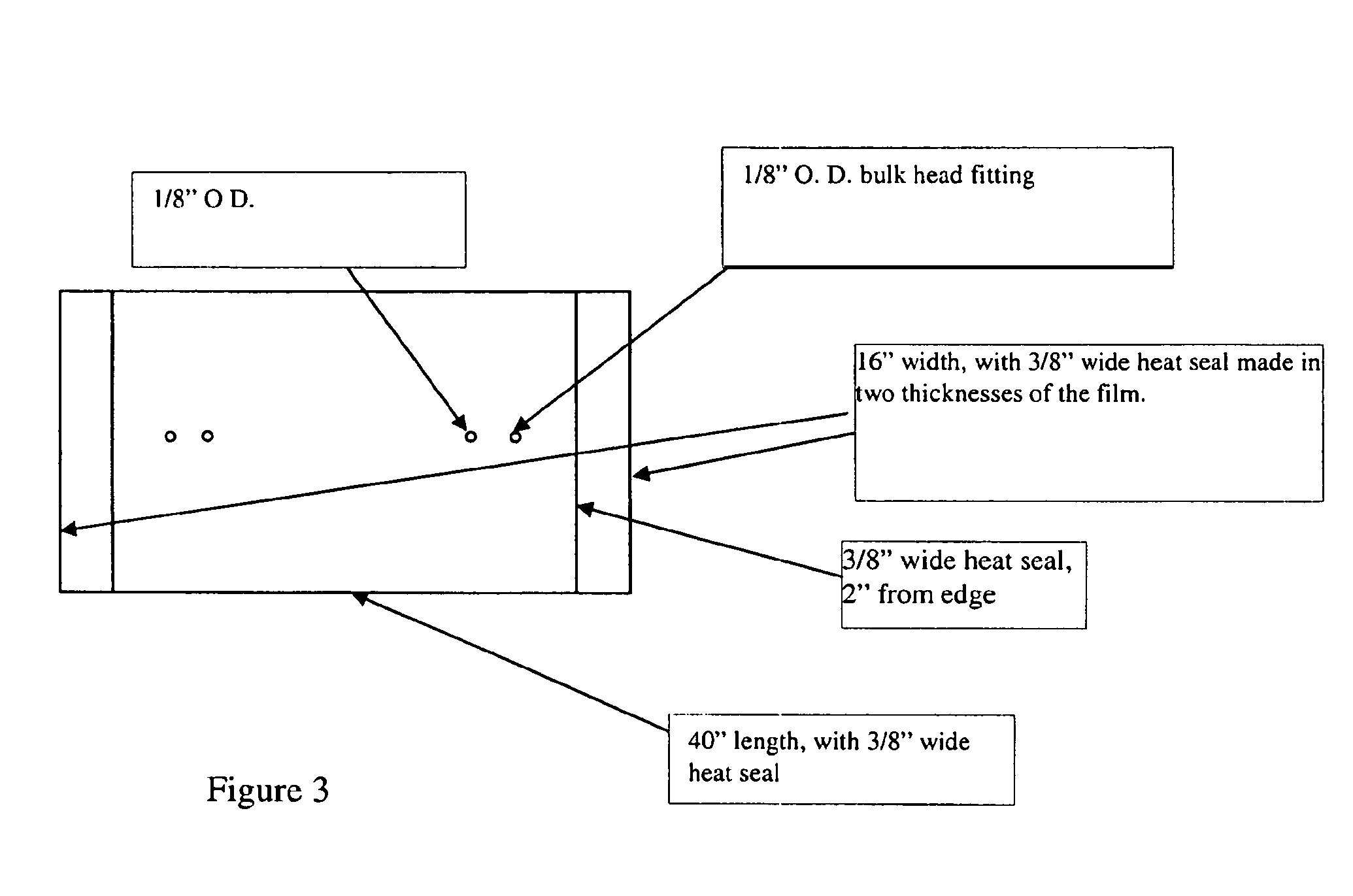
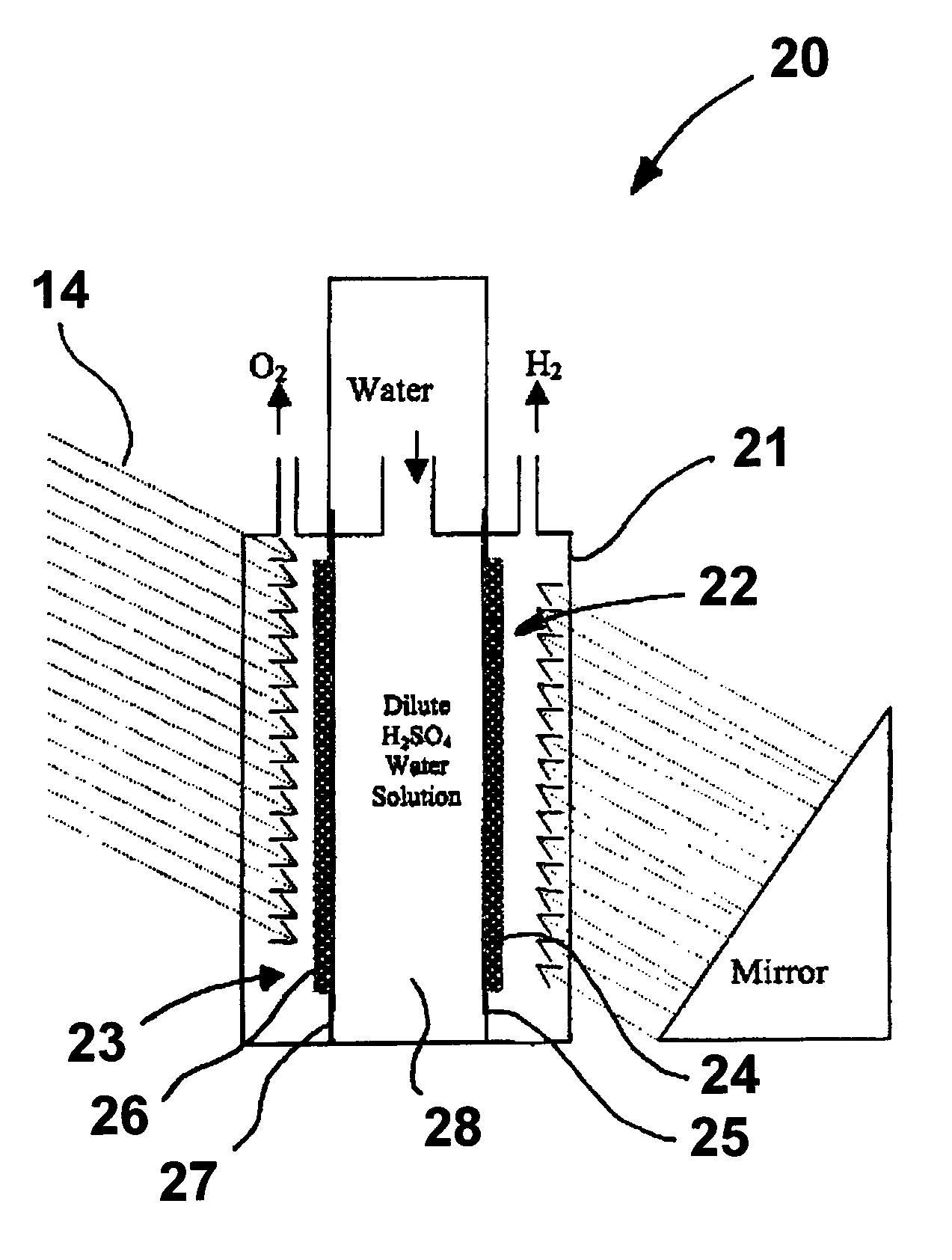
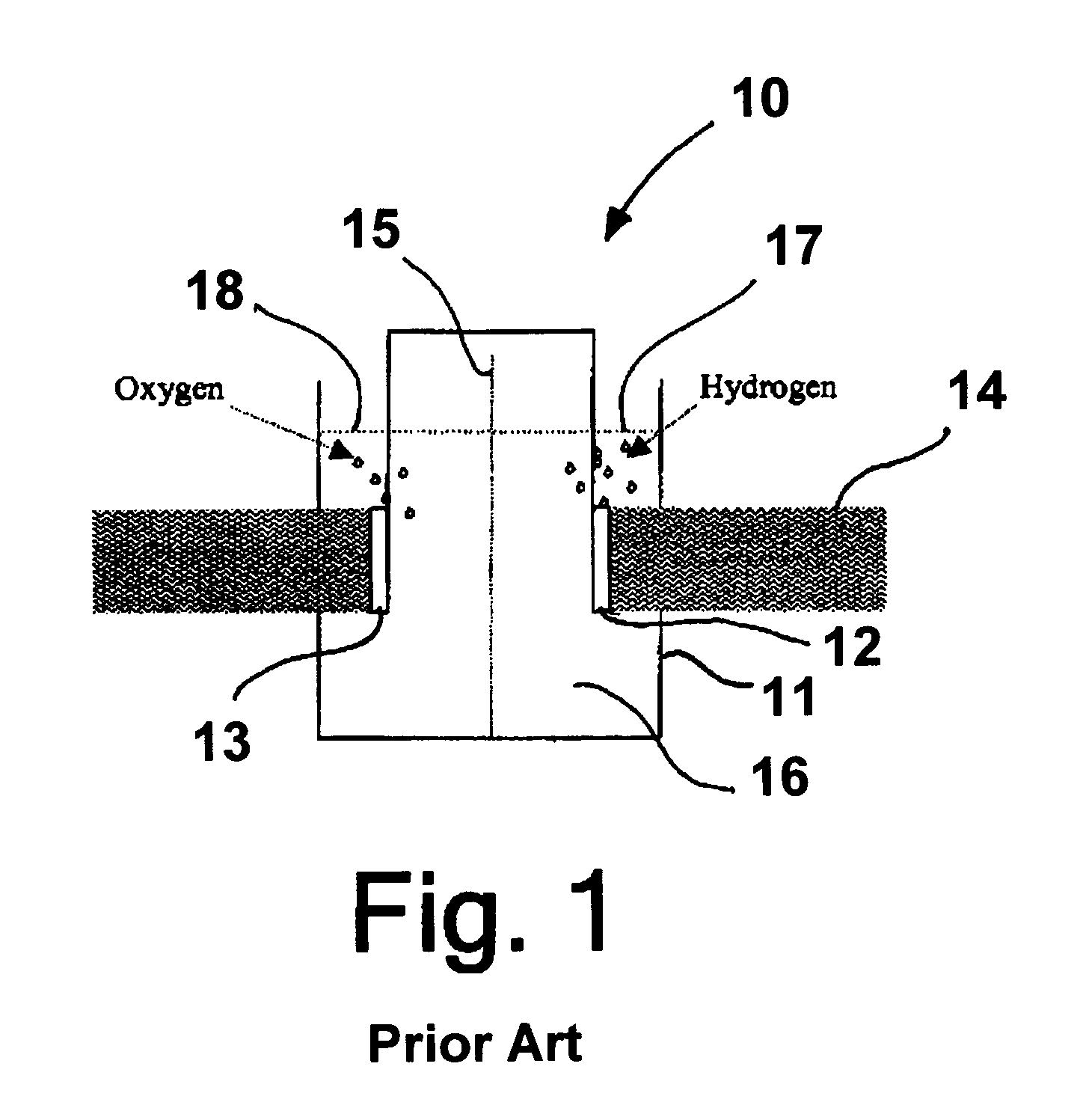
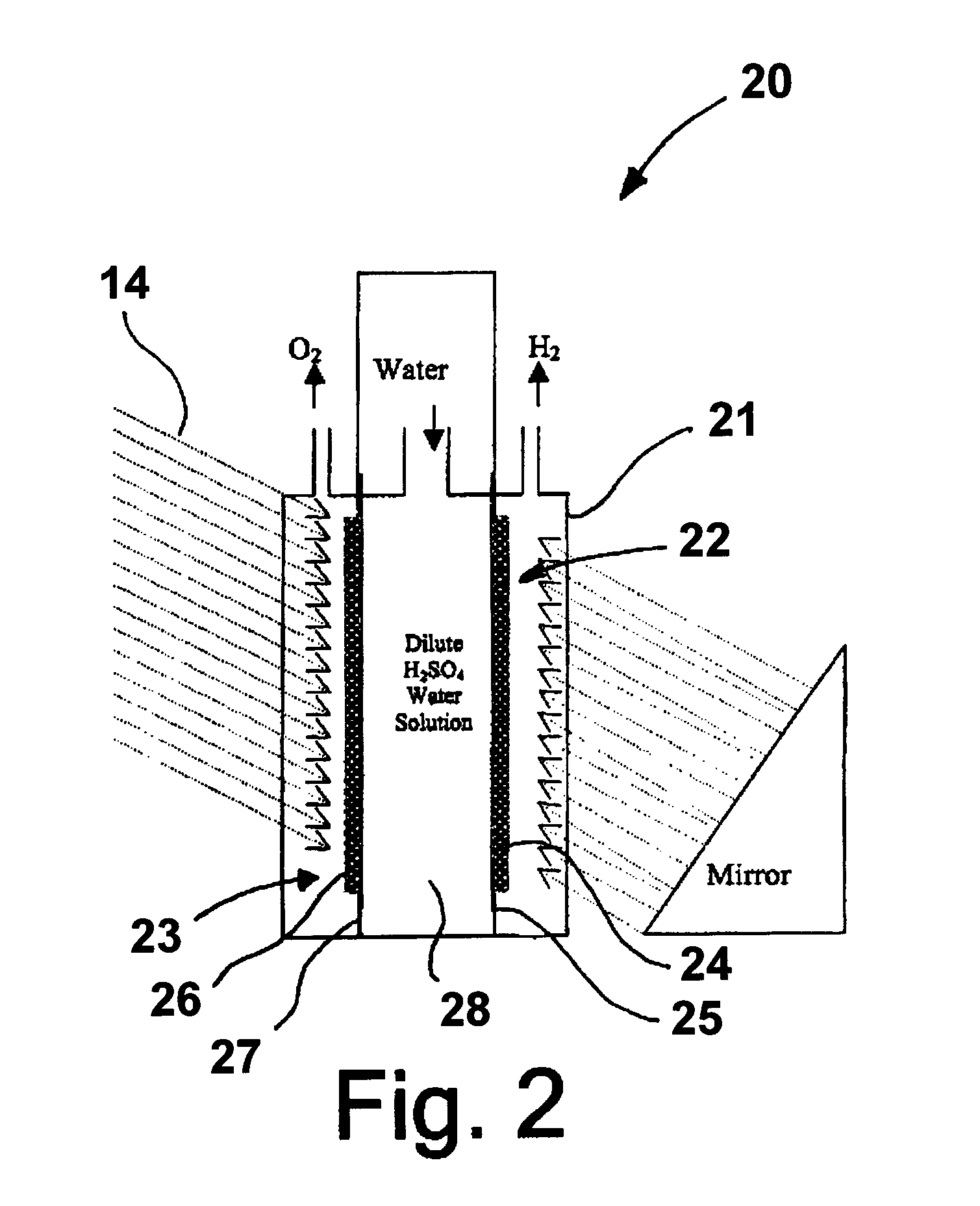
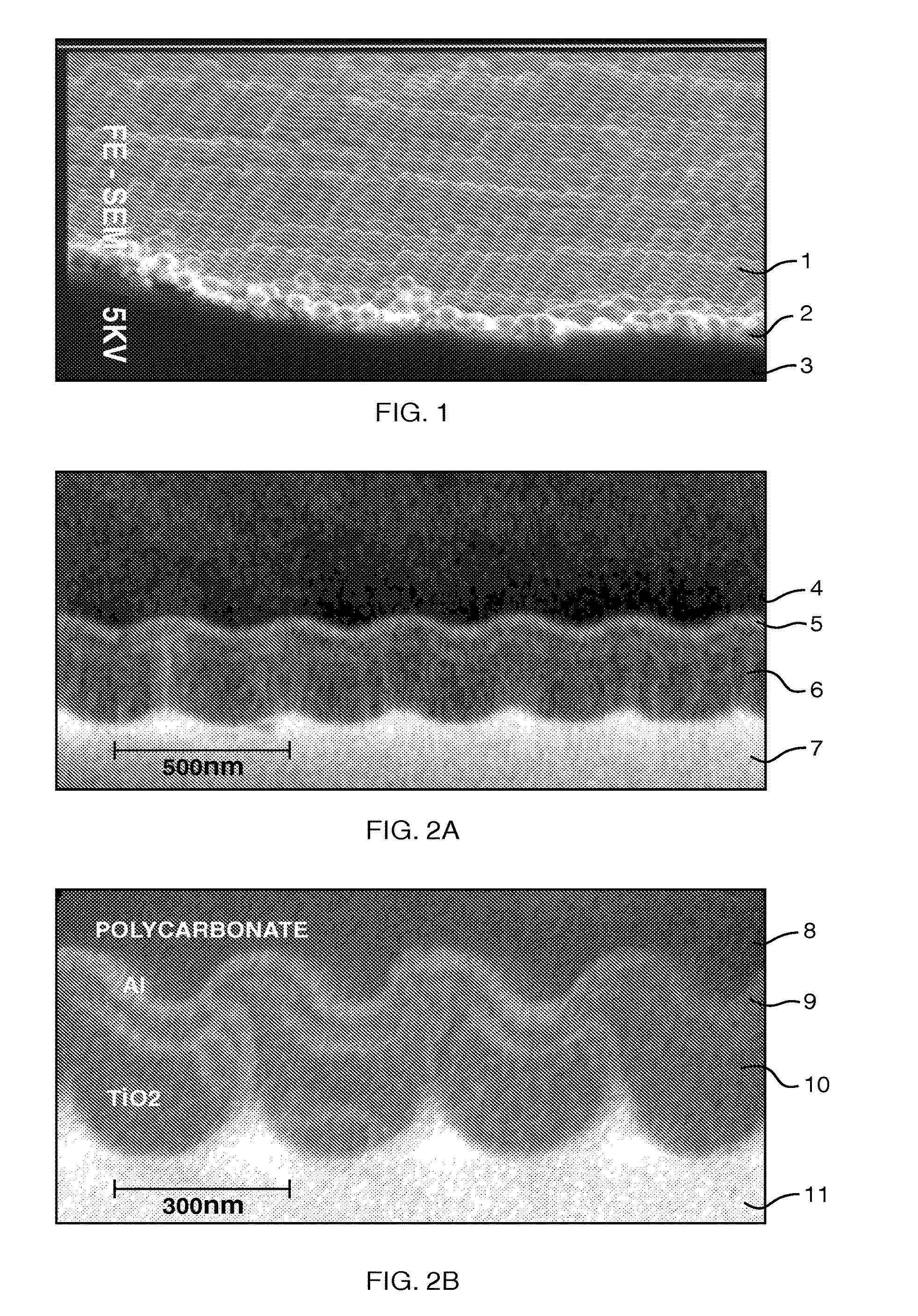
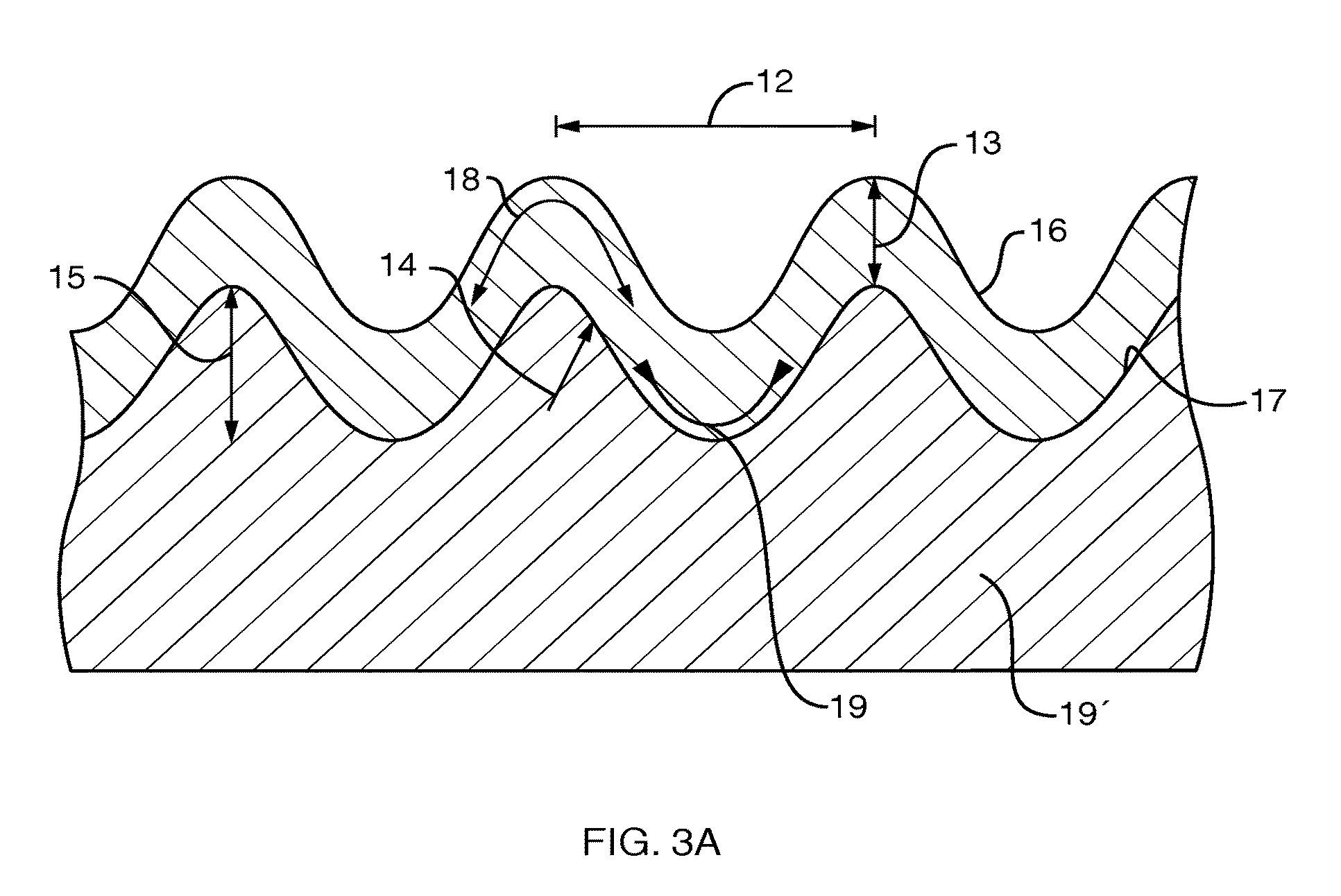
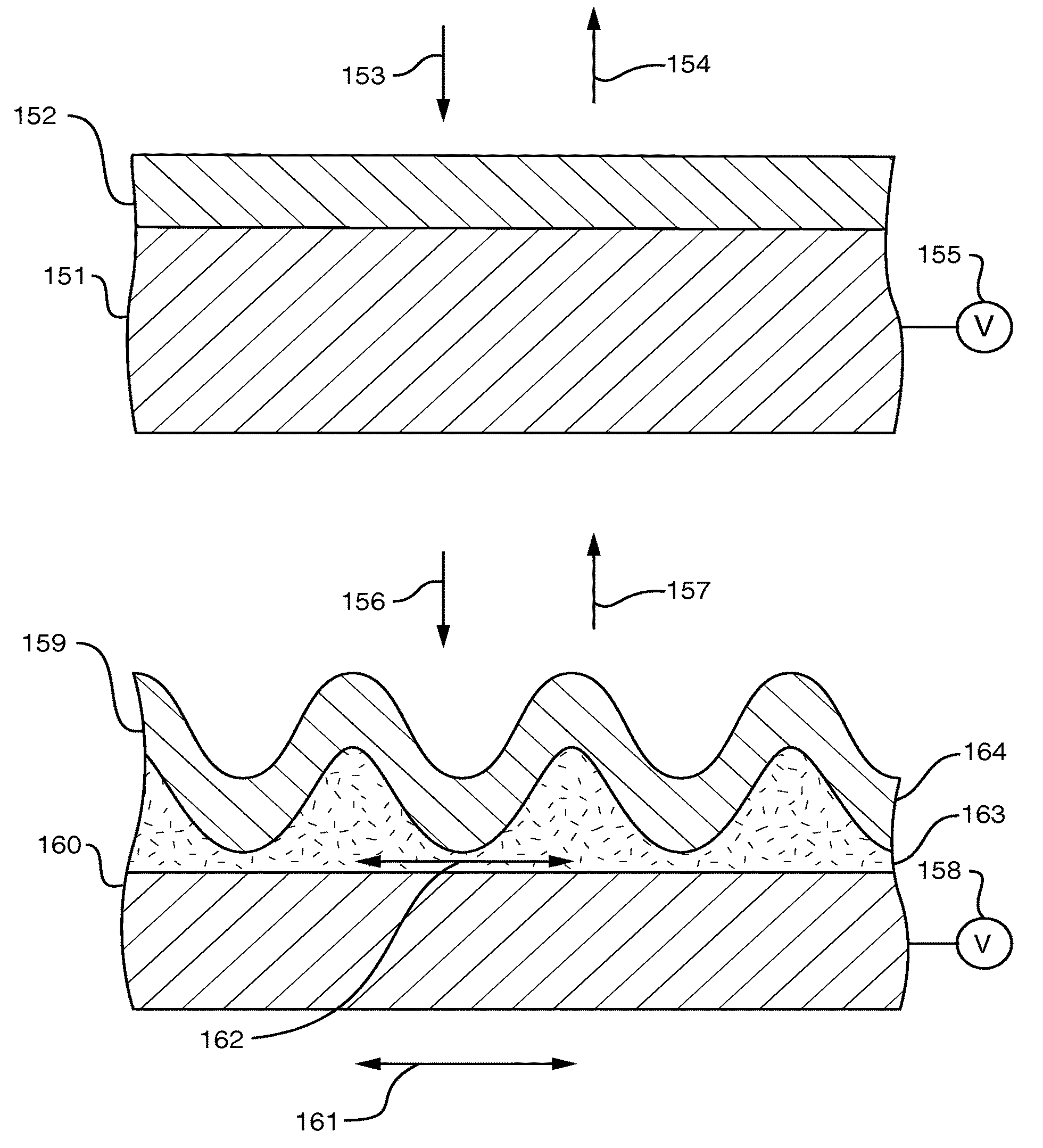
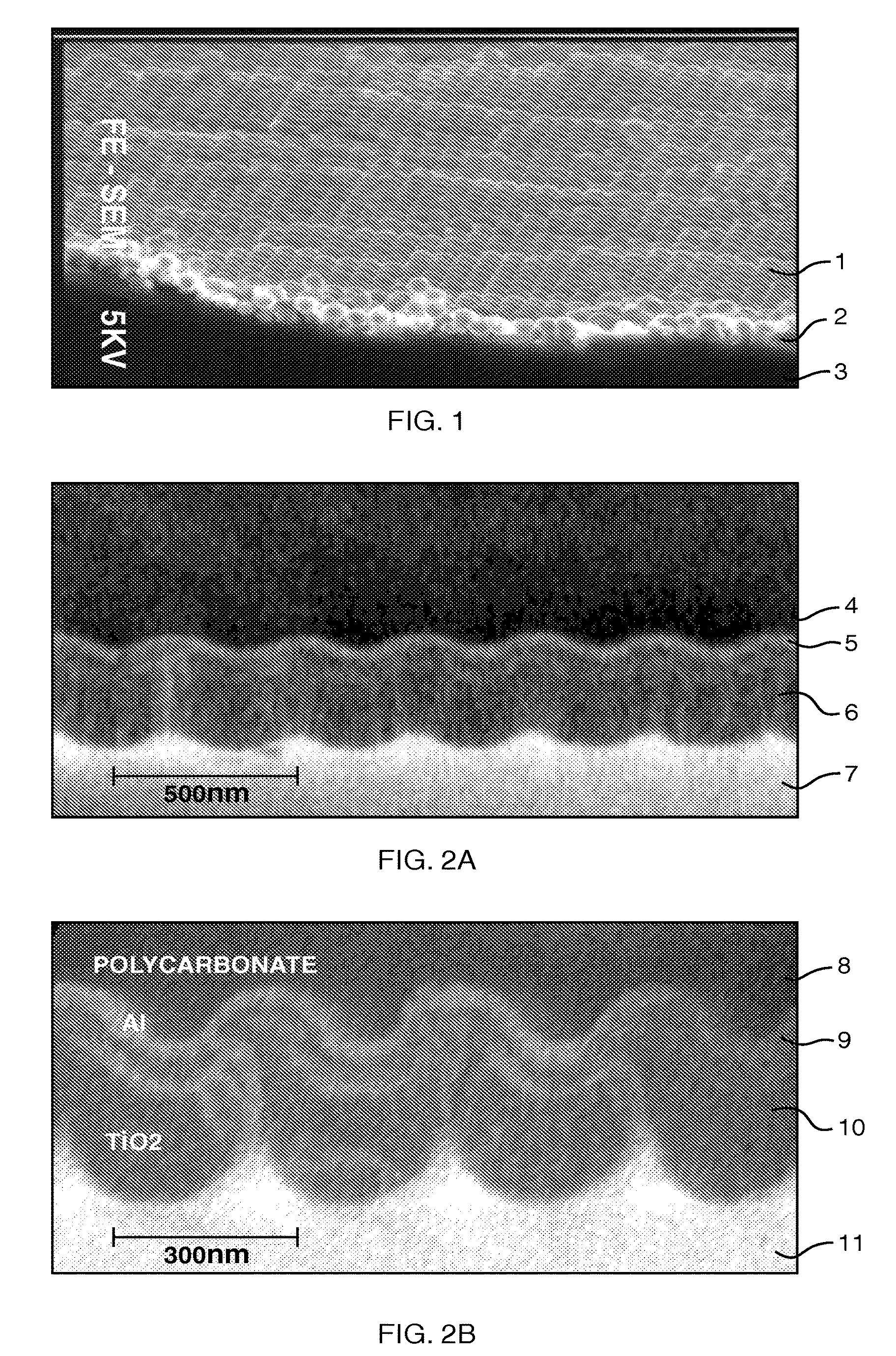
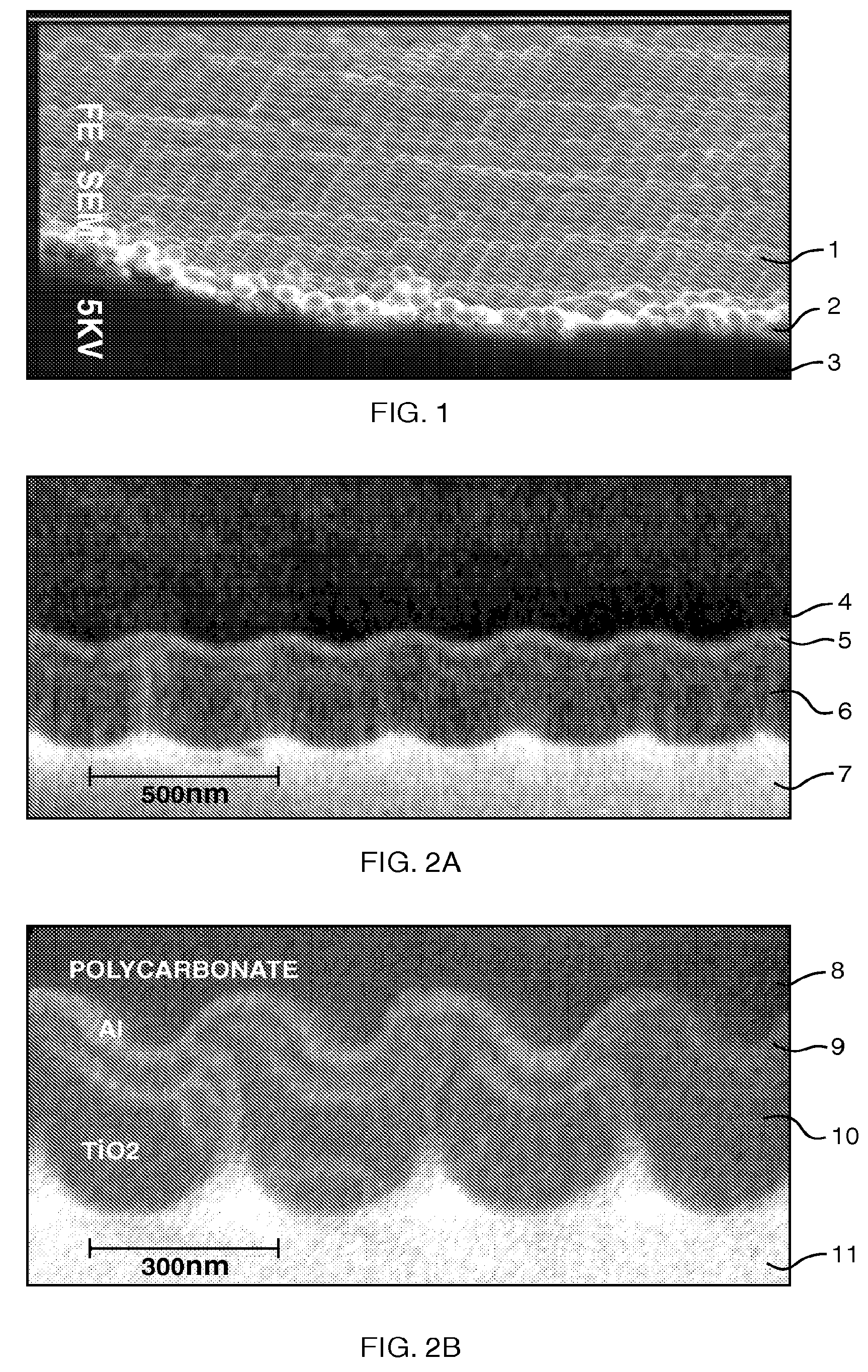
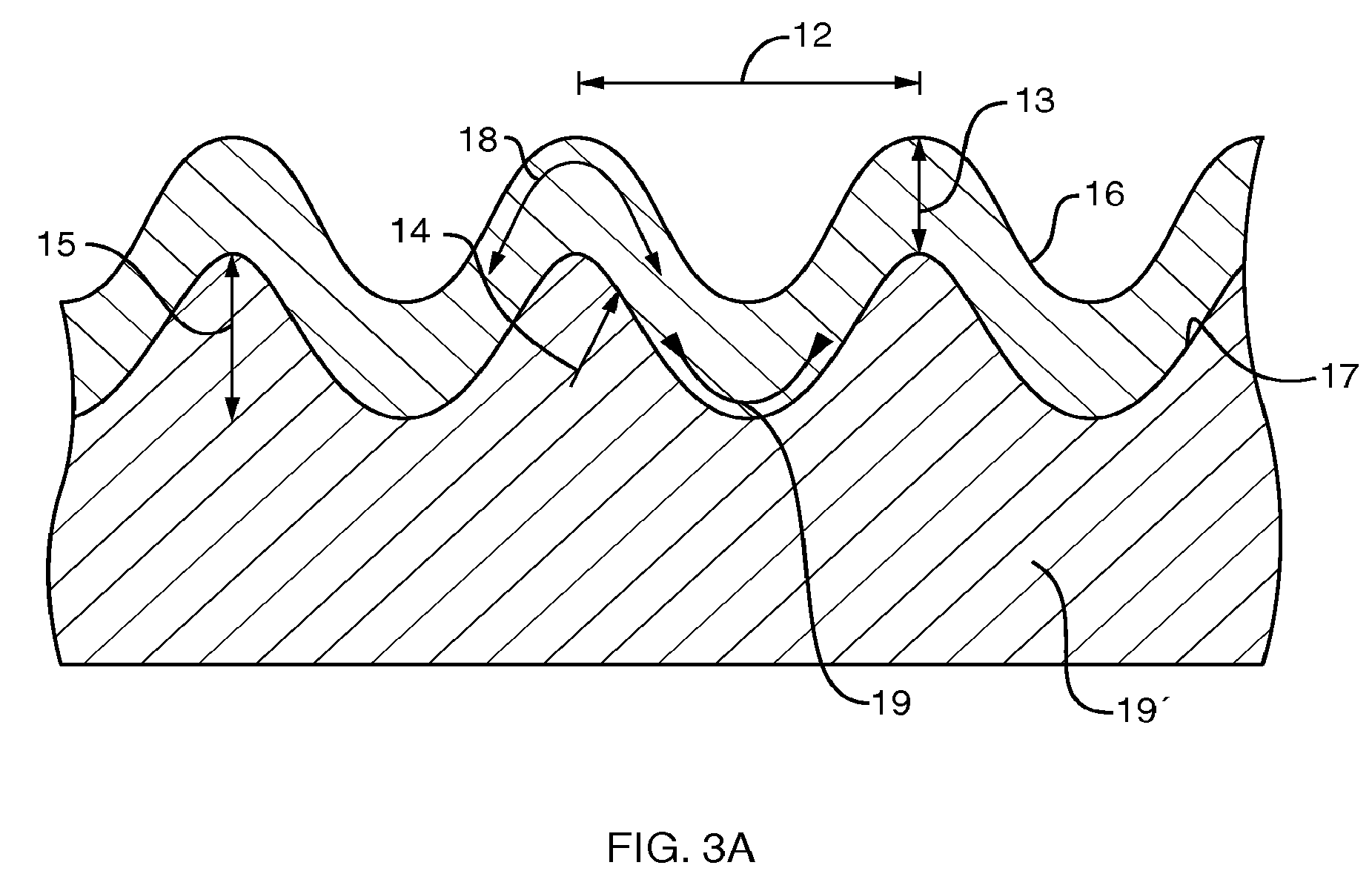
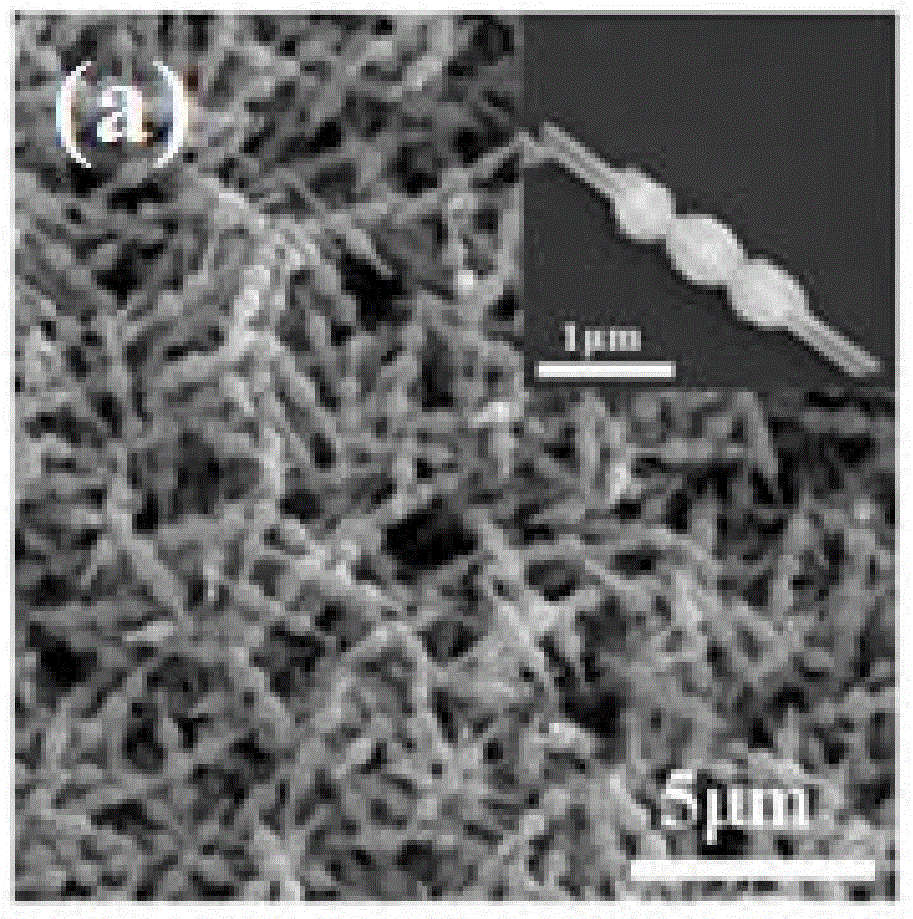
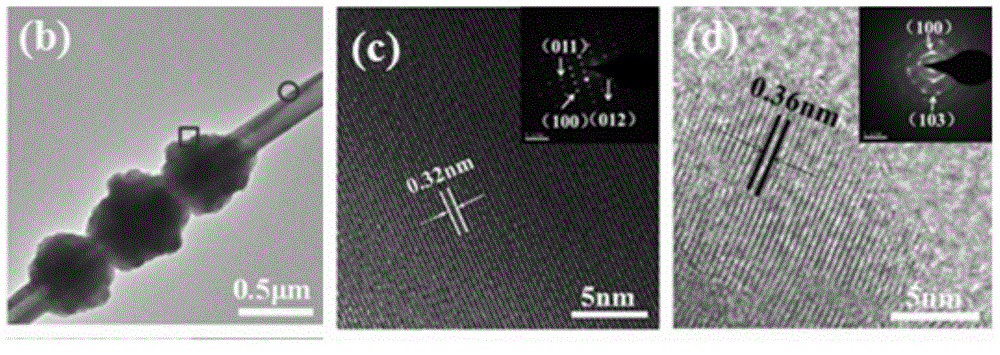
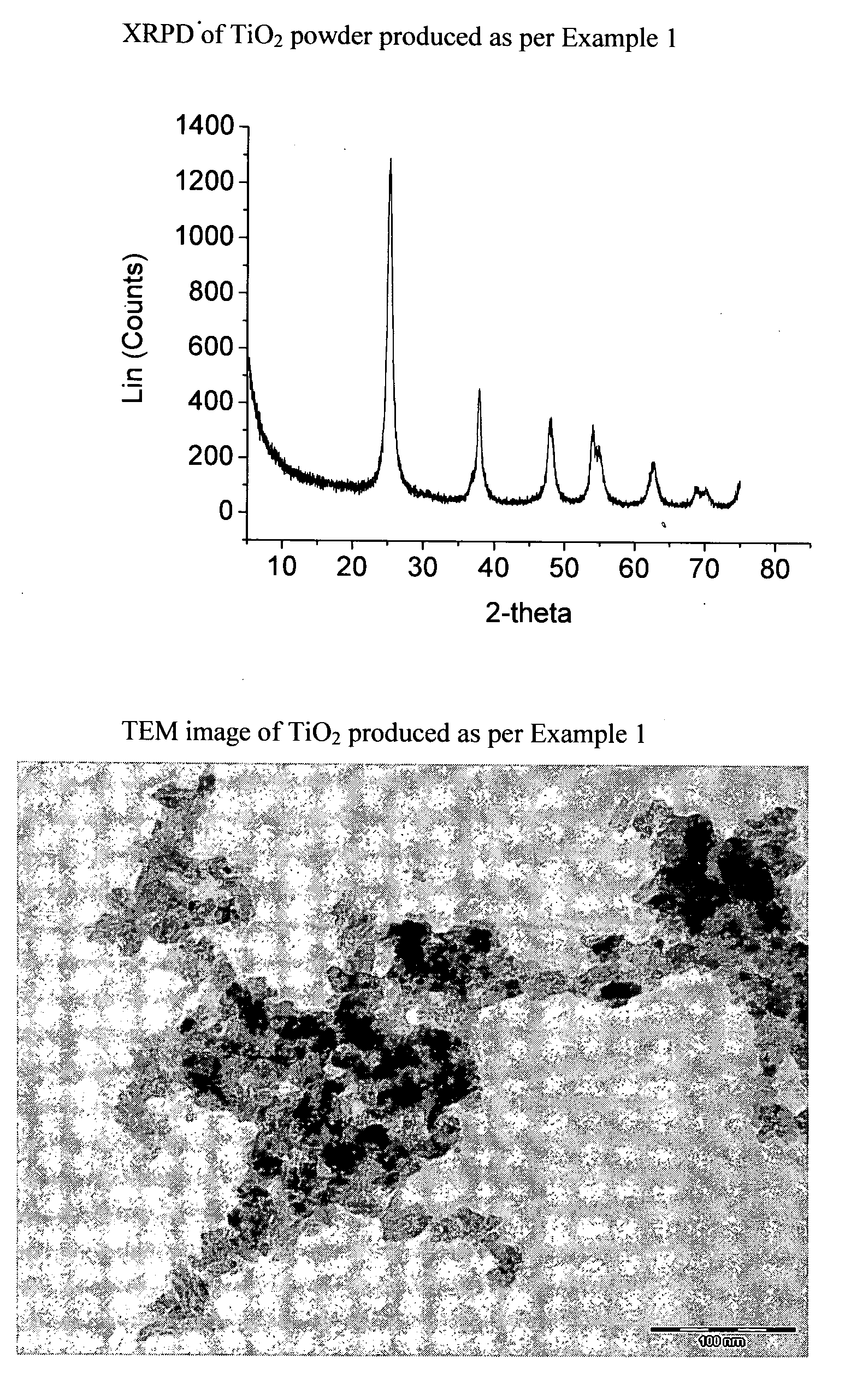
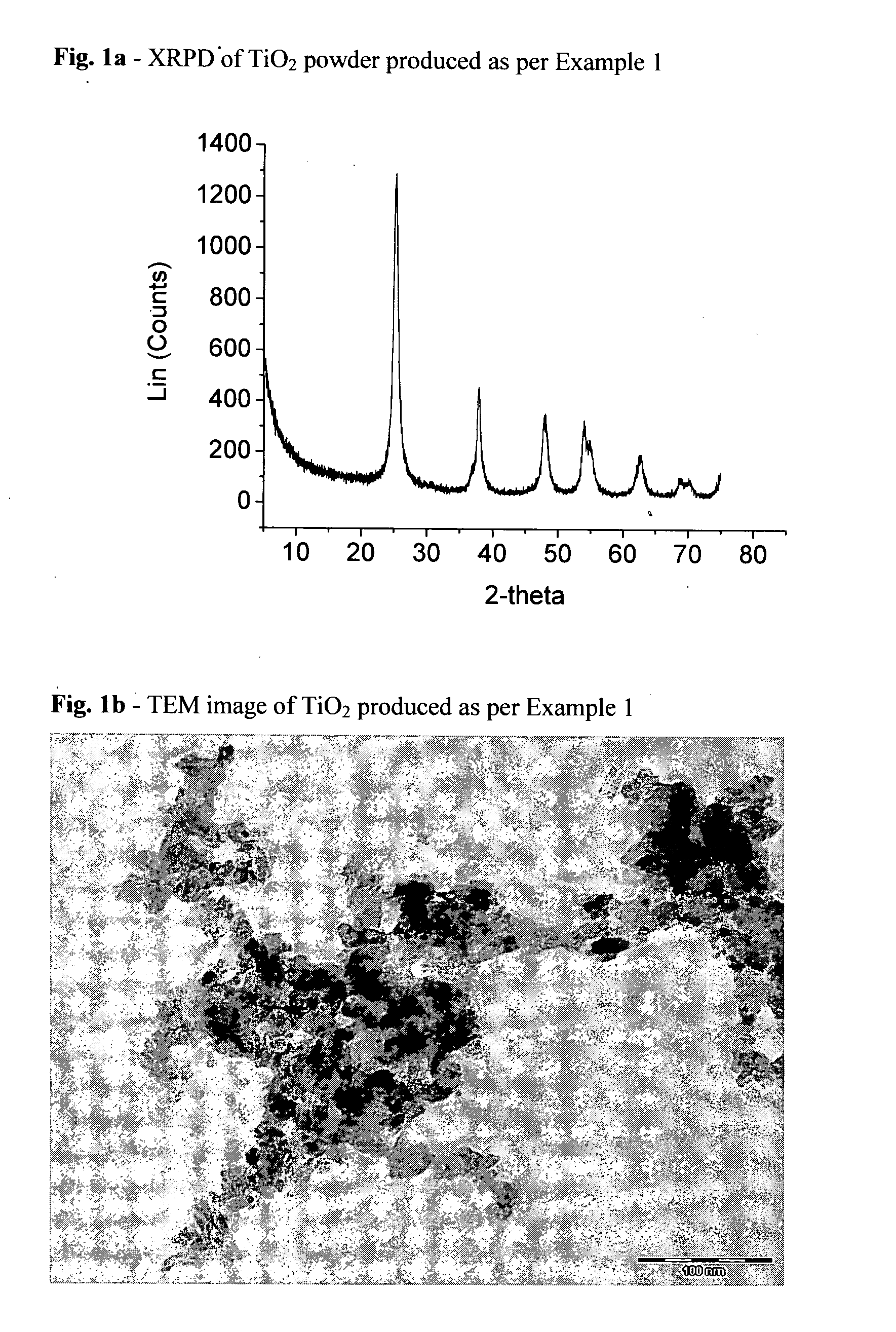
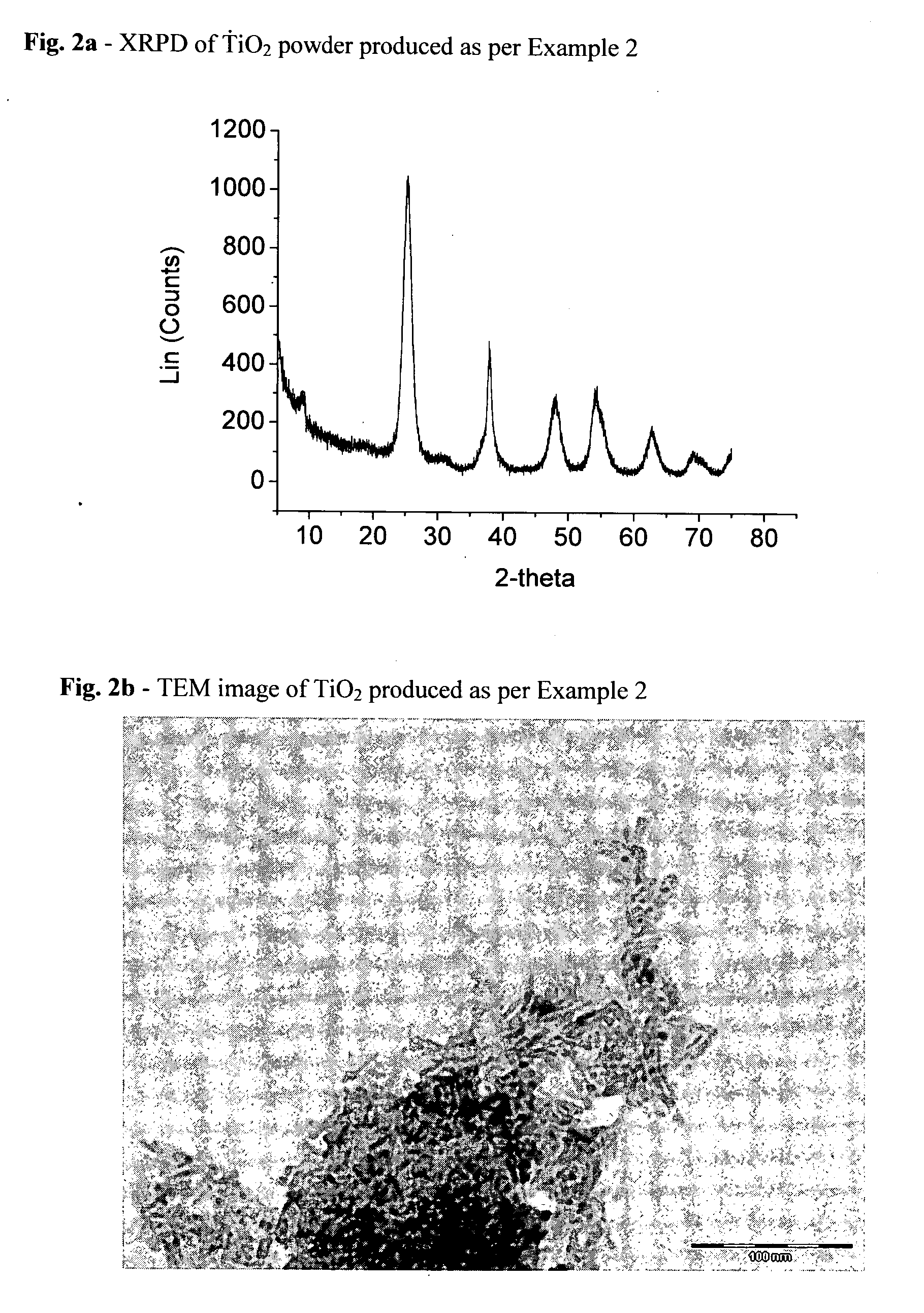
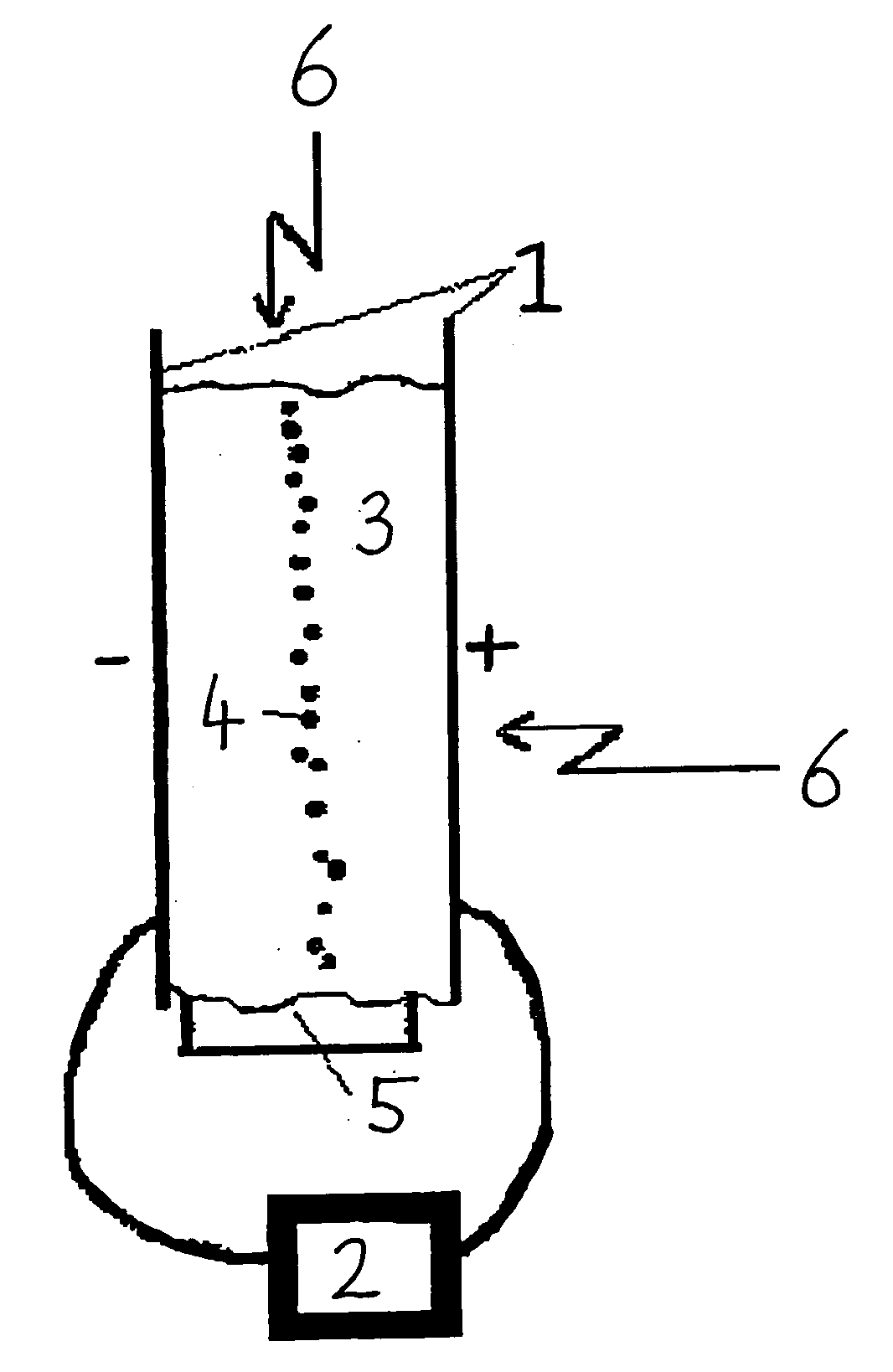
Patents
Literature
85 results about "Photoelectrolysis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
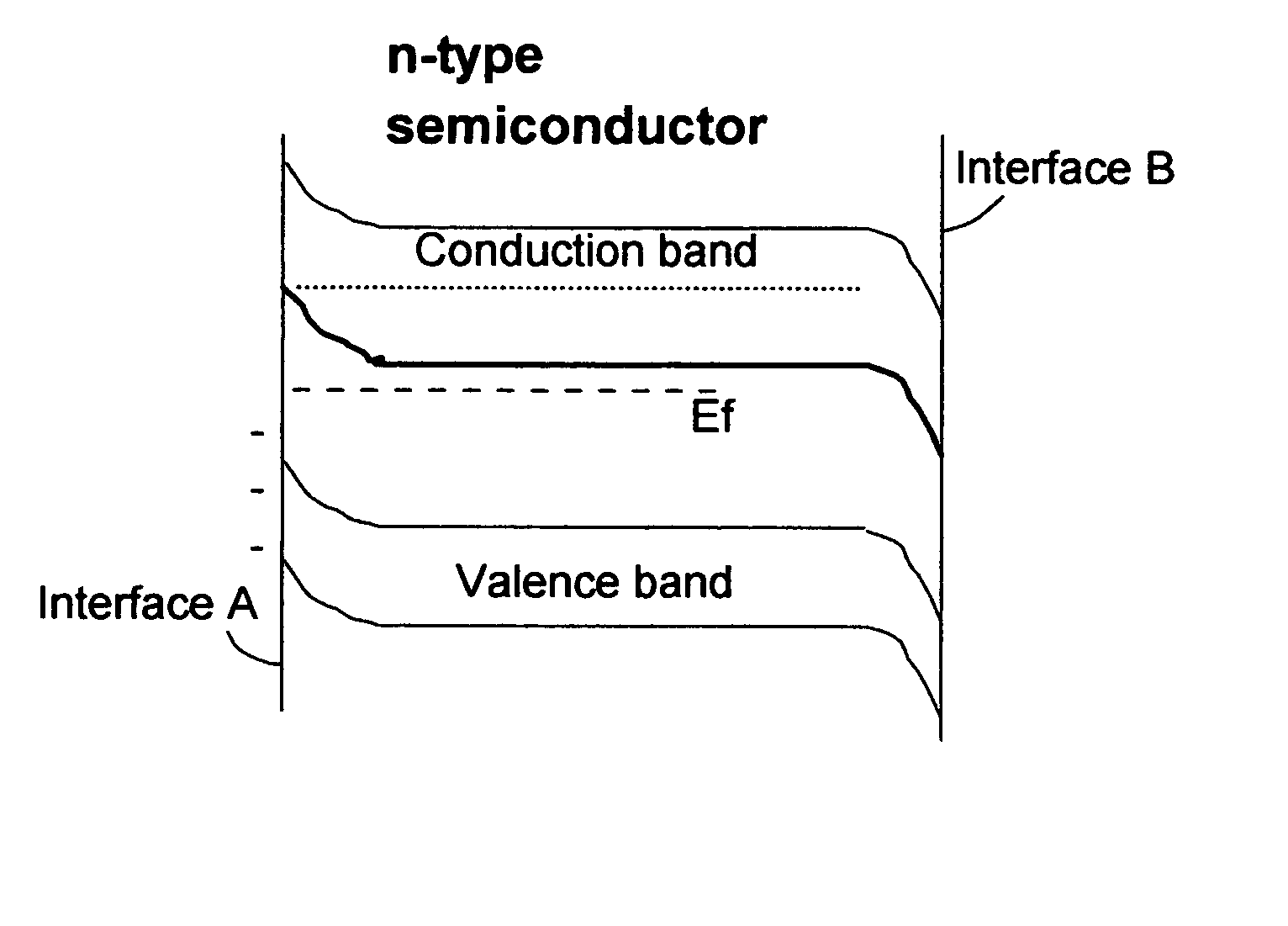
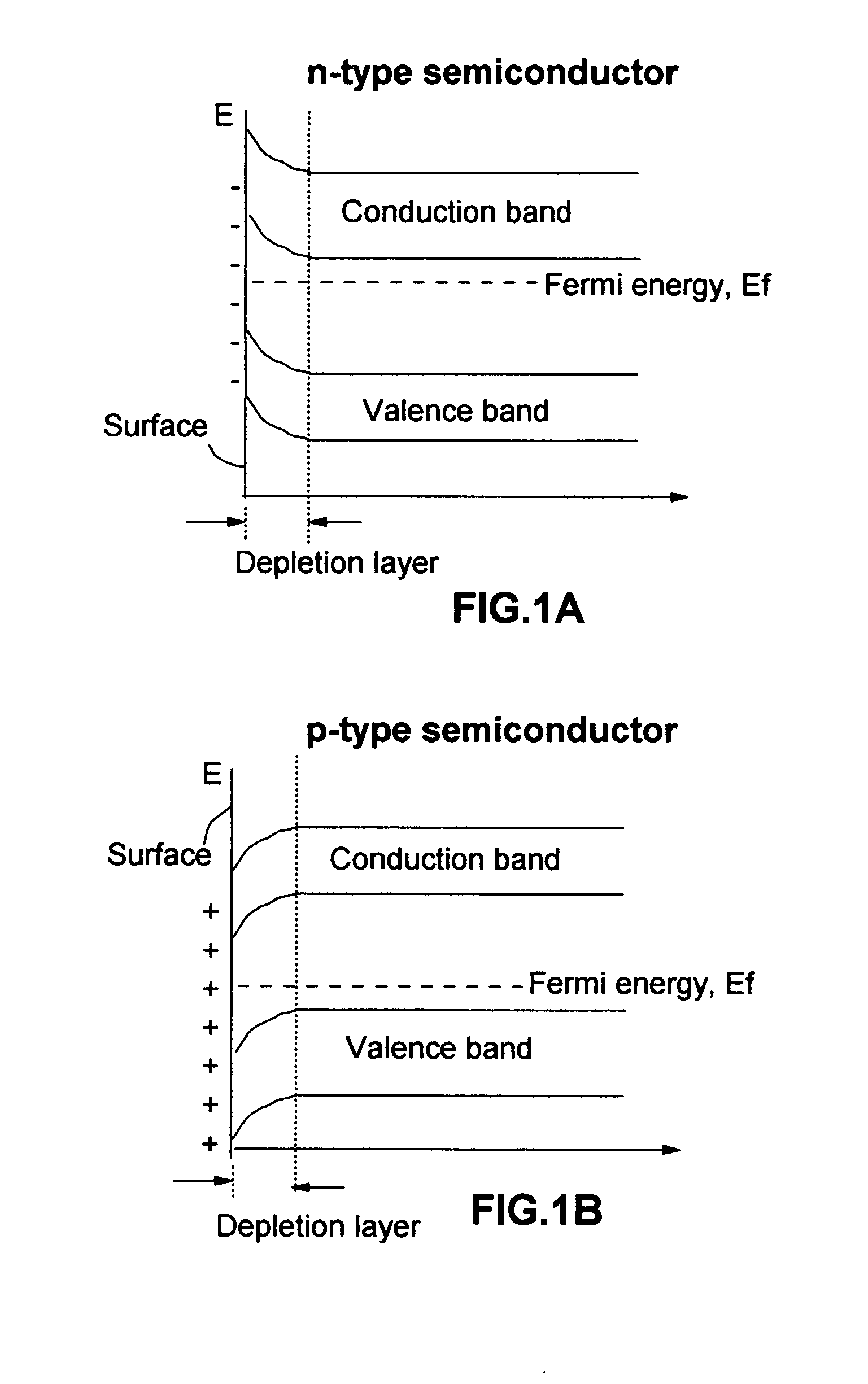
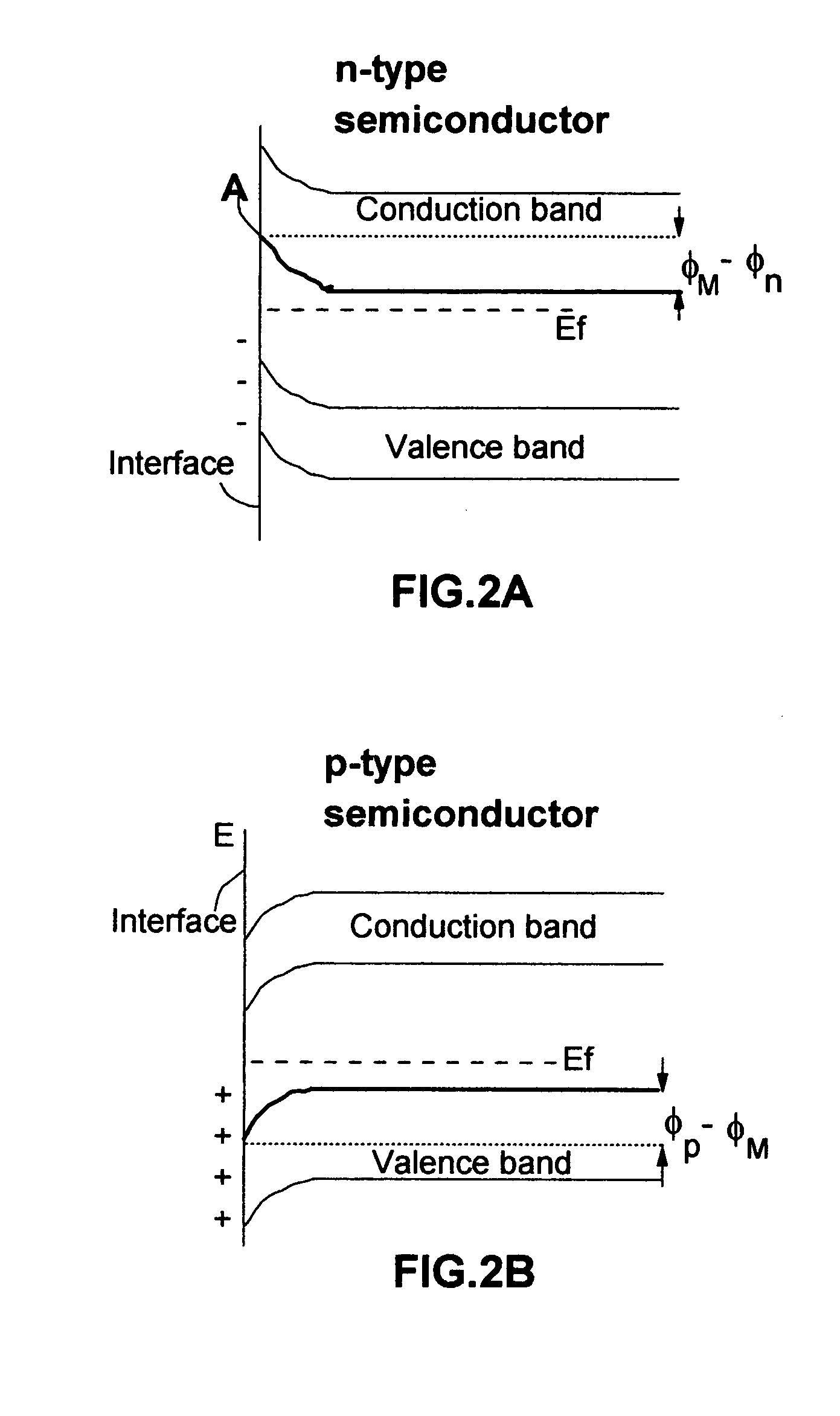
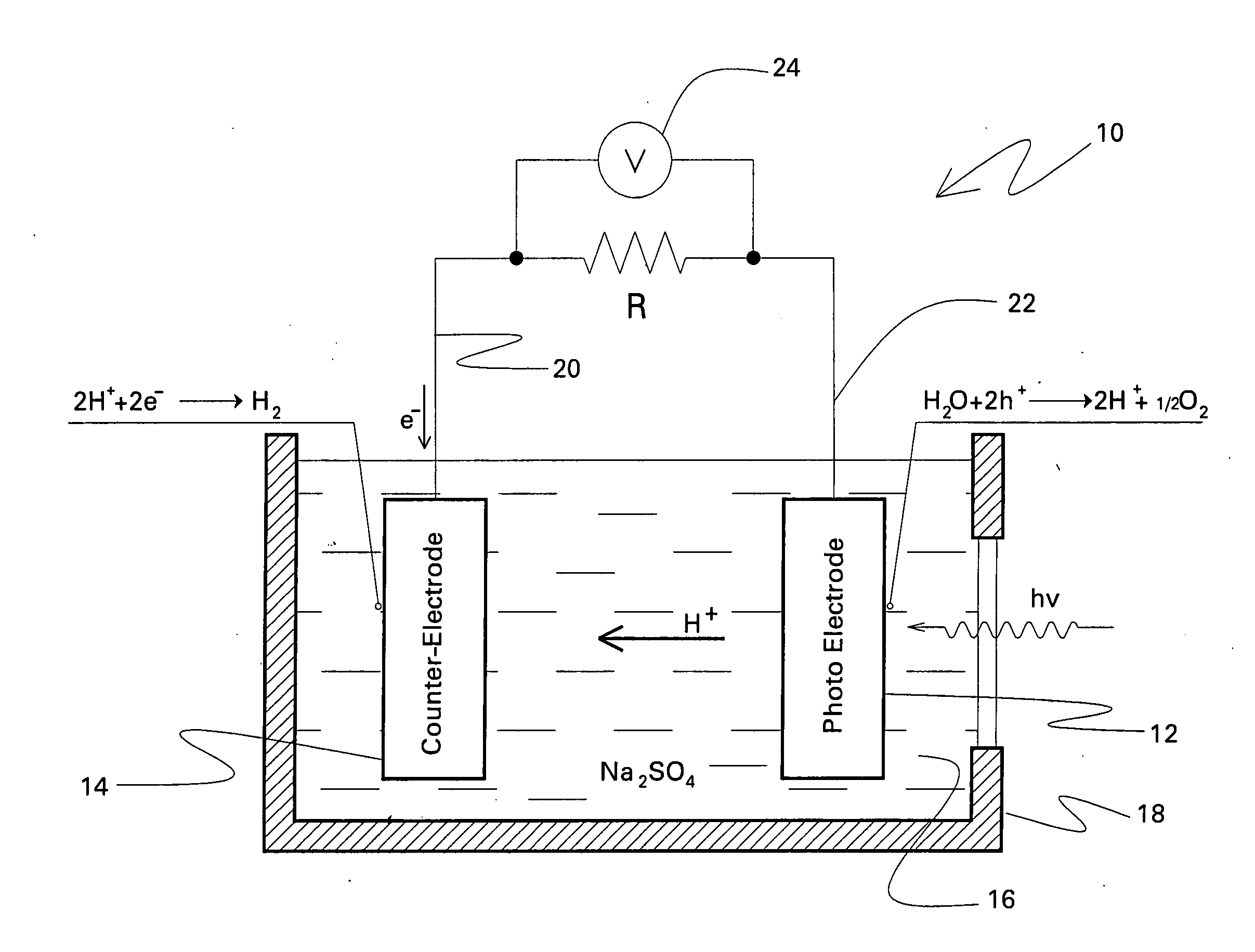
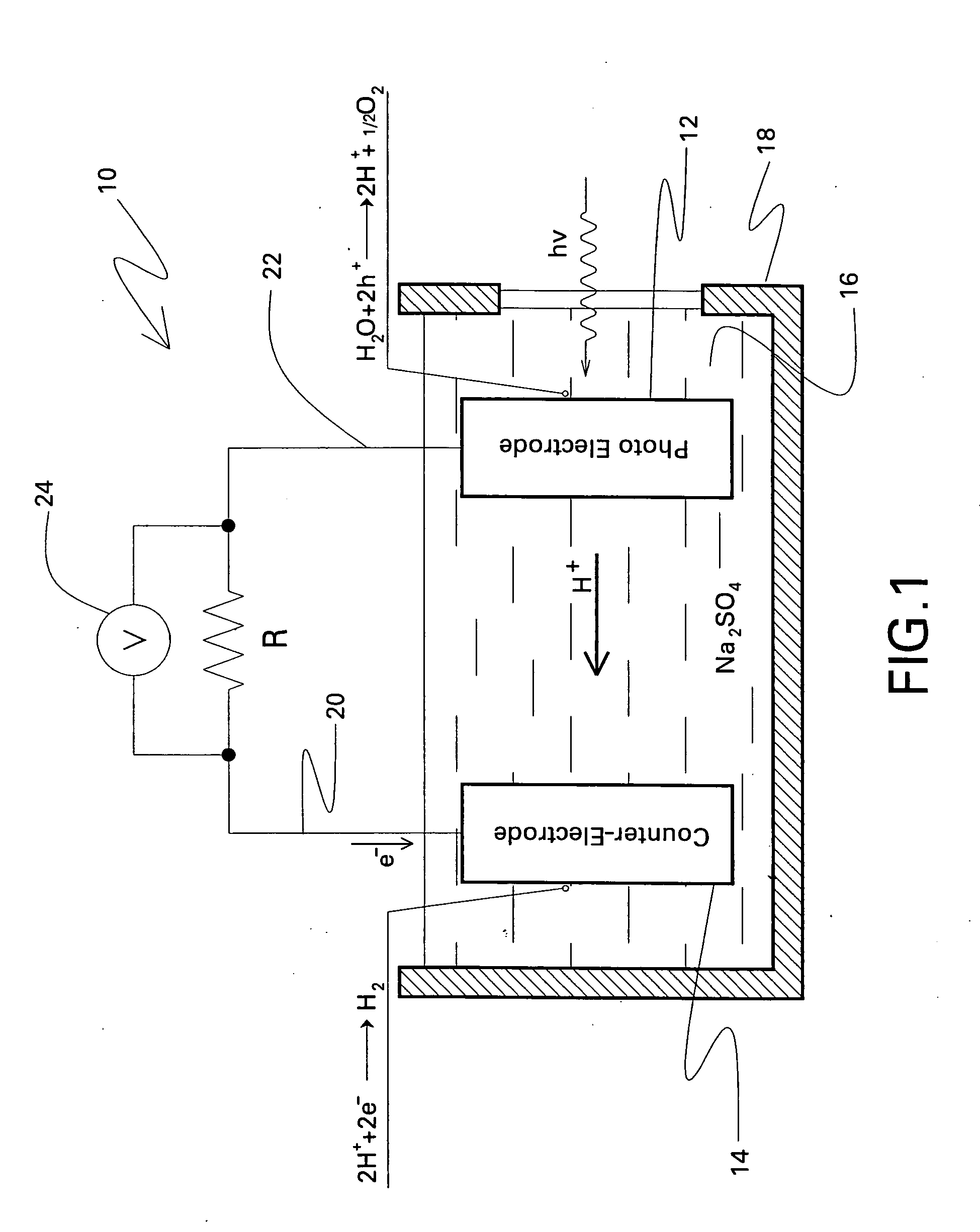
Photoelectrolysis, also known as water splitting, occurs in a photoelectrochemical cell when light is used as the energy source for the electrolysis of water, producing dihydrogen which can be used as a fuel. This process is one route to a "hydrogen economy", in which hydrogen fuel is produced efficiently and inexpensively from natural sources without using fossil fuels. In contrast, steam reforming usually or always uses a fossil fuel to obtain hydrogen. Photoelectrolysis is sometimes known colloquially as the hydrogen holy grail for its potential to yield a viable alternative to petroleum as a source of energy; such an energy source would supposedly come without the sociopolitically undesirable effects of extracting and using petroleum.
Photo-electrolytic catalyst systems and method for hydrogen production from water
InactiveUS20050051439A1Reduce probabilityIncrease chanceCellsMaterial nanotechnologyElectron holeHigh rate
A photo-electrolytic catalyst system which comprises two materials: (a) a semiconductor material with a non-zero energy gap Eg which, in response to an incident radiation having an energy greater than Eg, generates electron-hole pairs as charge carriers; and (b) a facilitating material in electronic contact with the semiconductor material to facilitate separation of the radiation-generated electrons from the holes to reduce the probability of charge carrier recombinations The catalyst makes use of both majority and minority charge carriers to promote photo-electrolysis reactions for producing hydrogen directly from water or an aqueous electrolyte at higher rates and improved efficiencies.
Owner:JANG BOR Z
Stress-induced bandgap-shifted semiconductor photoelectrolytic/photocatalytic/photovoltaic surface and method for making same
ActiveUS7485799B2Improve efficiencyIncrease photocatalytic surface areaLight-sensitive devicesInternal combustion piston enginesStress inducedHydrogen
Titania is a semiconductor and photocatalyst that is also chemically inert. With its bandgap of 3.0, to activate the photocatalytic property of titania requires light of about 390 nm wavelength, which is in the ultra-violet (UV), where sunlight is very low in intensity. A method and devices are disclosed wherein stress is induced and managed in a thin film of titania in order to shift and lower the bandgap energy into the longer wavelengths that are more abundant in sunlight. Applications of this stress-induced bandgap-shifted titania photocatalytic surface include photoelectrolysis for production of hydrogen gas from water, photovoltaics for production of electricity, and photocatalysis for detoxification and disinfection.
Owner:NANOPTEK CORP
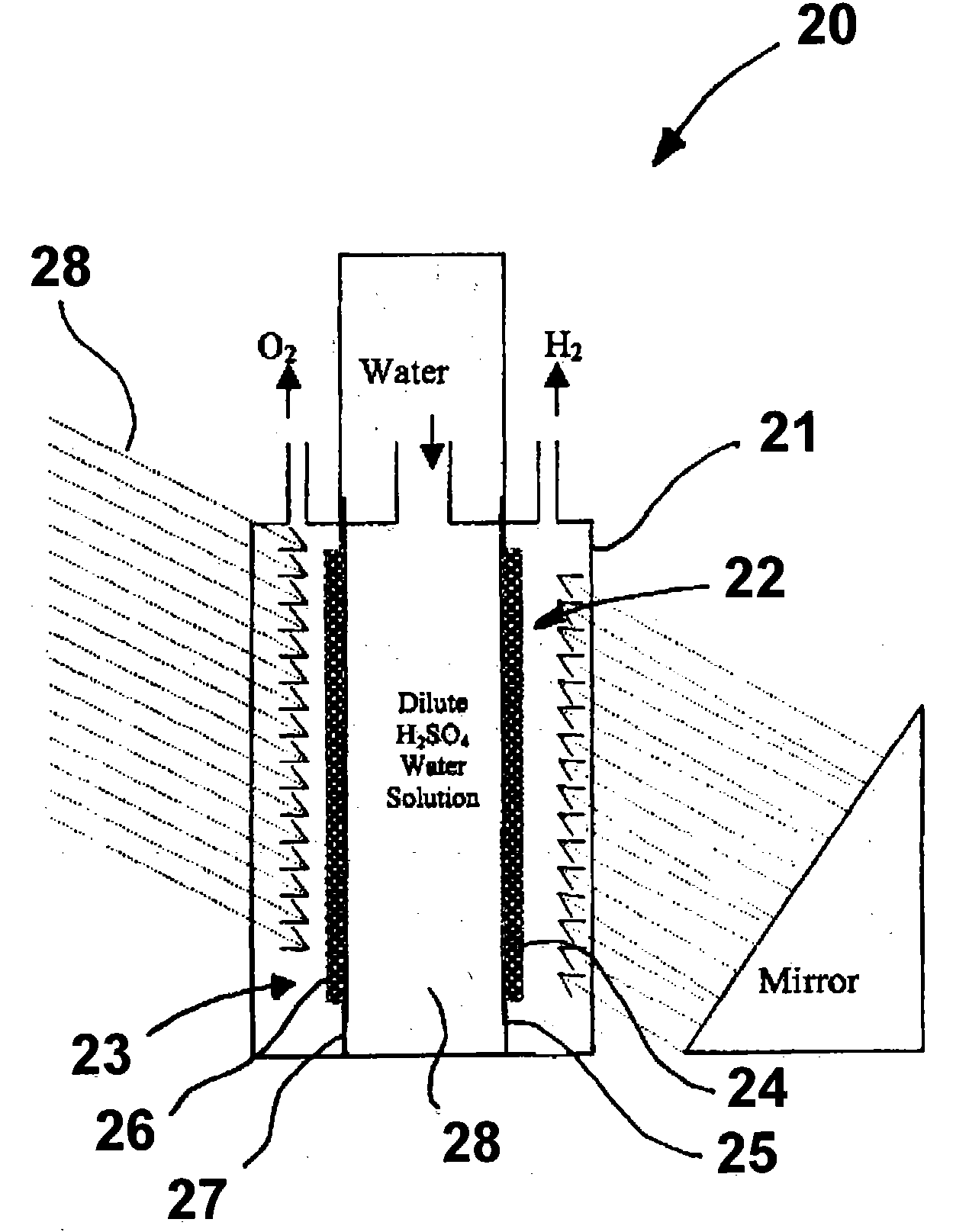
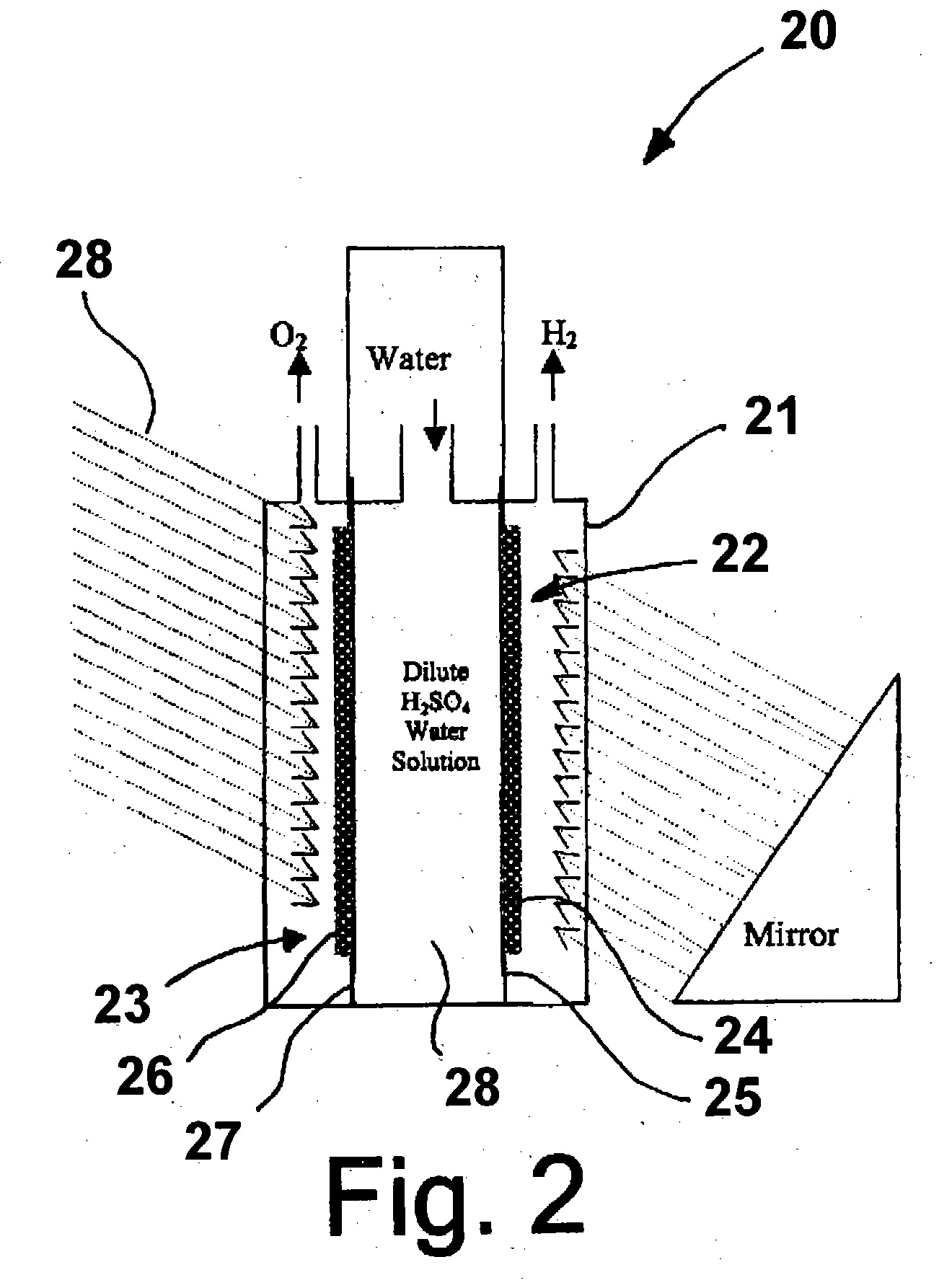
Hydrogen generator photovoltaic electrolysis reactor system
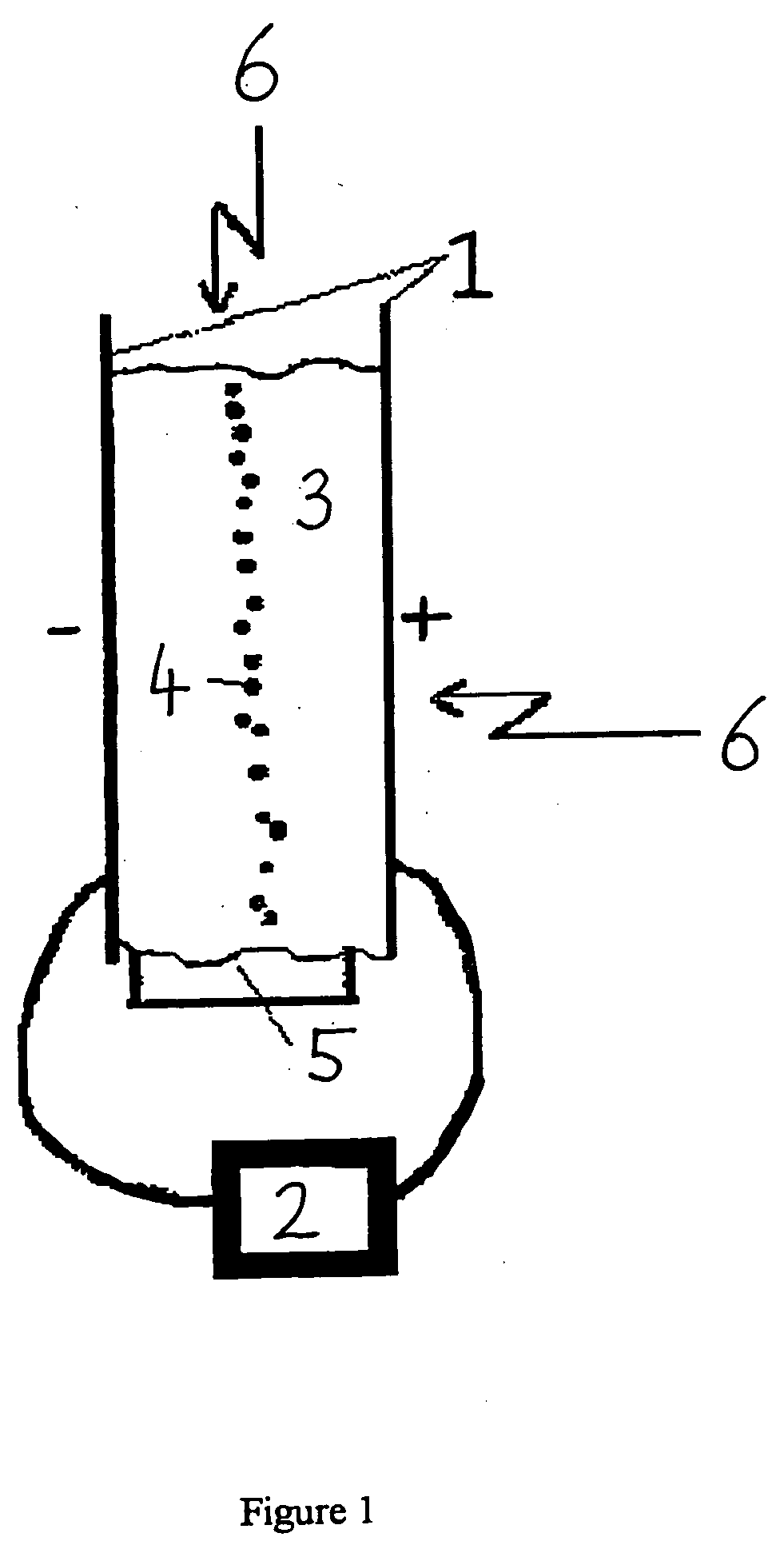
An apparatus for creating hydrogen from the disassociation of water using sunlight (photoelectrolysis) is provided. The system utilizes an aqueous fluid filled container which functions both to hold the water to be disassociated and as a light collecting lens. A photovoltaic module is positioned at a point to most efficiently accept the refracted light from the fluid filled container. A pair of electrodes which are coupled to the photovoltaic module are disposed within the fluid and configured to split the water into hydrogen and oxygen.
Owner:GM GLOBAL TECH OPERATIONS LLC
Photoelectrolysis of water using proton exchange membranes
ActiveUS7037414B2Improve efficiencyImprove stabilityMachining electrodesCellsElectro catalystWater use
A photoelectrochemical cell which includes a light transmissive enclosure, a semiconductor photoanode disposed within the light transmissive enclosure, a semiconductor photocathode disposed within the light transmissive enclosure, and an electrolytic solution disposed entirely between the semiconductor photoanode and the semiconductor photocathode. This is achieved by the use of semiconductor photoelectrodes (photoanodes and photocathodes) which include a proton exchange membrane having an electrolyte facing surface in contact with the electrolytic solution and a light transmissive wall facing surface, and having a photo electro-catalyst disposed on the light transmissive wall facing surface.
Owner:GAS TECH INST
Hydrogen generator photovoltaic electrolysis reactor system
An apparatus for creating hydrogen from the disassociation of water using sunlight (photoelectrolysis) is provided. The system utilizes an aqueous fluid filled container which functions both to hold the water to be disassociated and as a light collecting lens. A photovoltaic module is positioned at a point to most efficiently accept the refracted light from the fluid filled container. A pair of electrodes which are coupled to the photovoltaic module are disposed within the fluid and configured to split the water into hydrogen and oxygen.
Owner:GM GLOBAL TECH OPERATIONS LLC
TiO2/BiVO4 photo-anode material and preparation method thereof
InactiveCN104988533AEfficient separationEfficient decompositionElectrodesNano structuringLattice defects
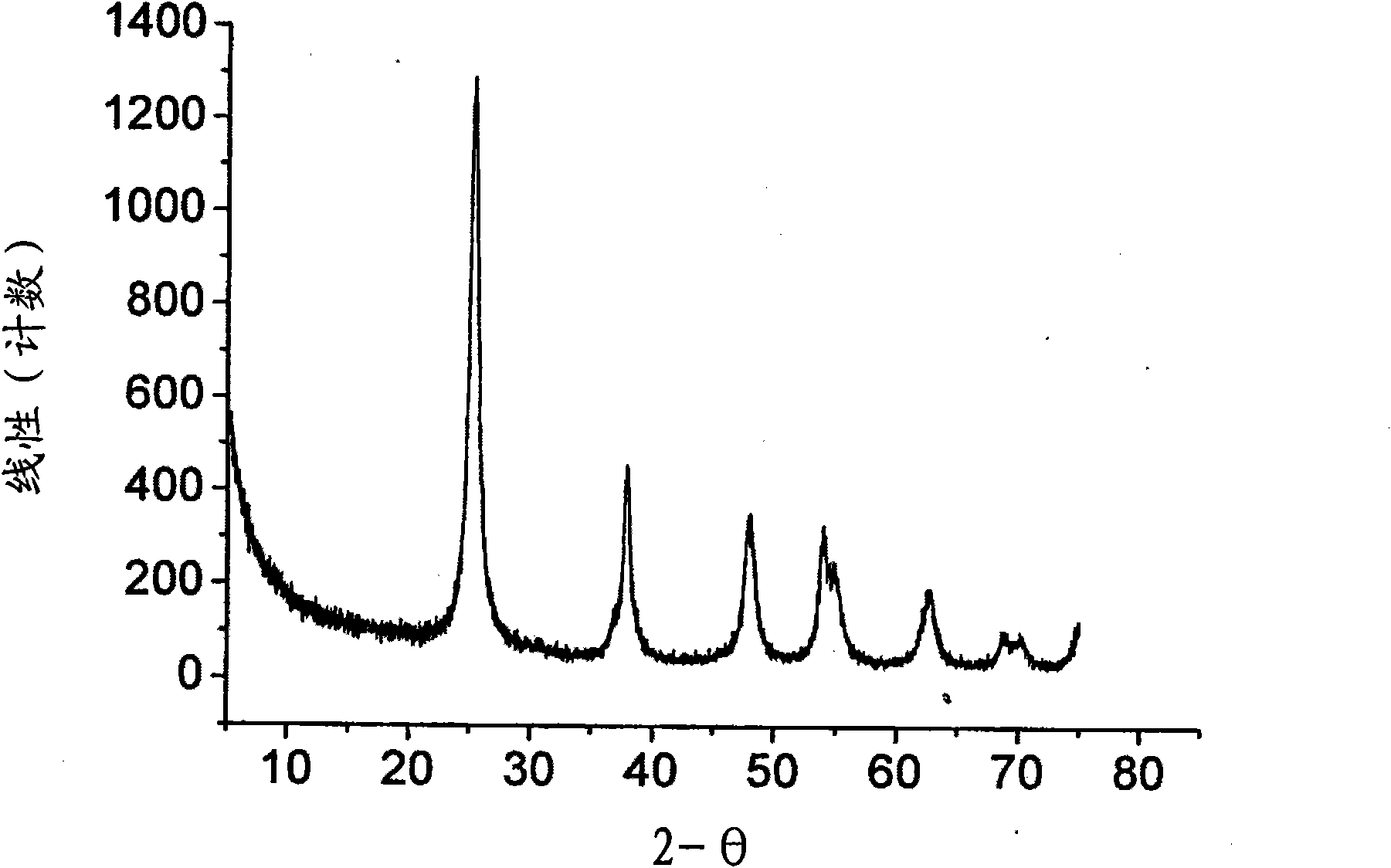
The invention discloses a TiO2 / BiVO4 photo-anode material which comprises a substrate, a TiO2 nano-rod array perpendicularly grown on the surface of the substrate, and a BiVO4 nano-particle layer deposited on the surface of the TiO2 nano-rod array. Through adoption of the TiO2 / BiVO4 photo-anode material, the water photoelectrolysis property is improved; compared with other water photoelectrolysis materials, the TiO2 / BiVO4 photo-anode material effectively overcomes the lattice defect of an interface layer, reduces the composition of photo-generated electrons and hole pairs, improves the own stability, expands the absorption spectrum range of visible light, promotes the effective separation of the photo-generated electrons and the hole pairs, realizes the synchronous reaction of hydrogen production and oxygen production, ensures that the ratio of the hydrogen yield to the oxygen yield is close to 2: 1, and is a relatively ideal water photoelectrolysis material. Moreover, the invention further discloses a preparation method of the TiO2 / BiVO4 photo-anode material. The preparation method has the characteristics that the nano-structure control is easy to realize technically, the prepared binary nano-rod array is excellent in crystallization property, and the interface quality is relatively high.
Owner:HUBEI UNIV
Preparation method for alpha-Fe2O3 photoanode applied to photoelectrolysis
InactiveCN103726090APrecise thickness controlQuick and friendly to prepareElectrolytic inorganic material coatingElectrodesGraphiteSaturated calomel electrode
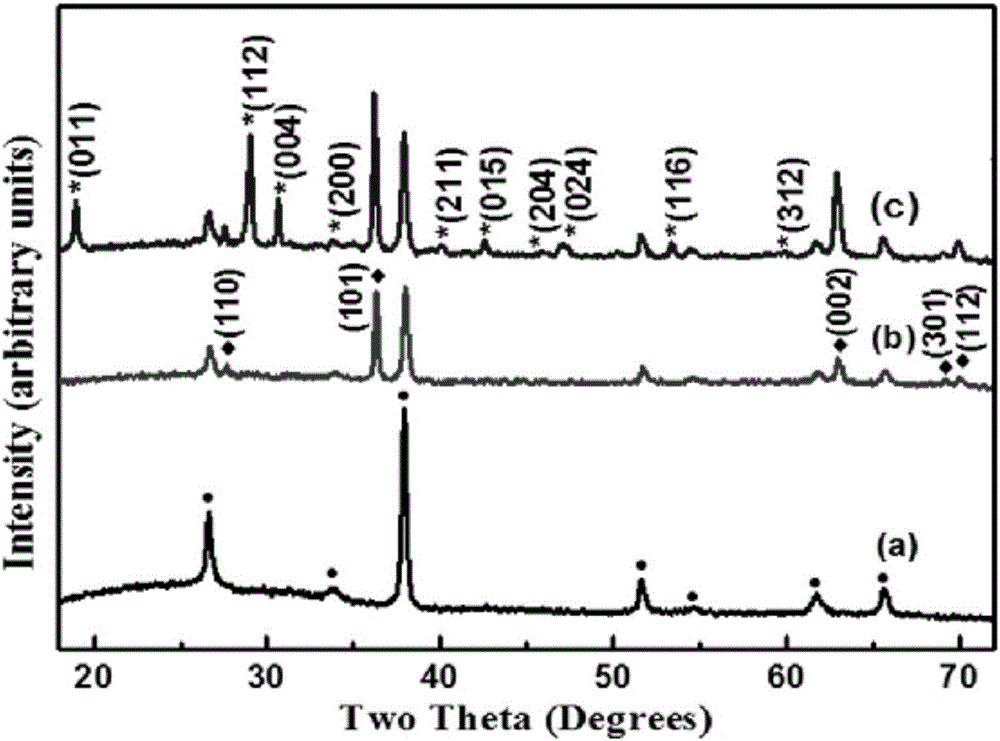
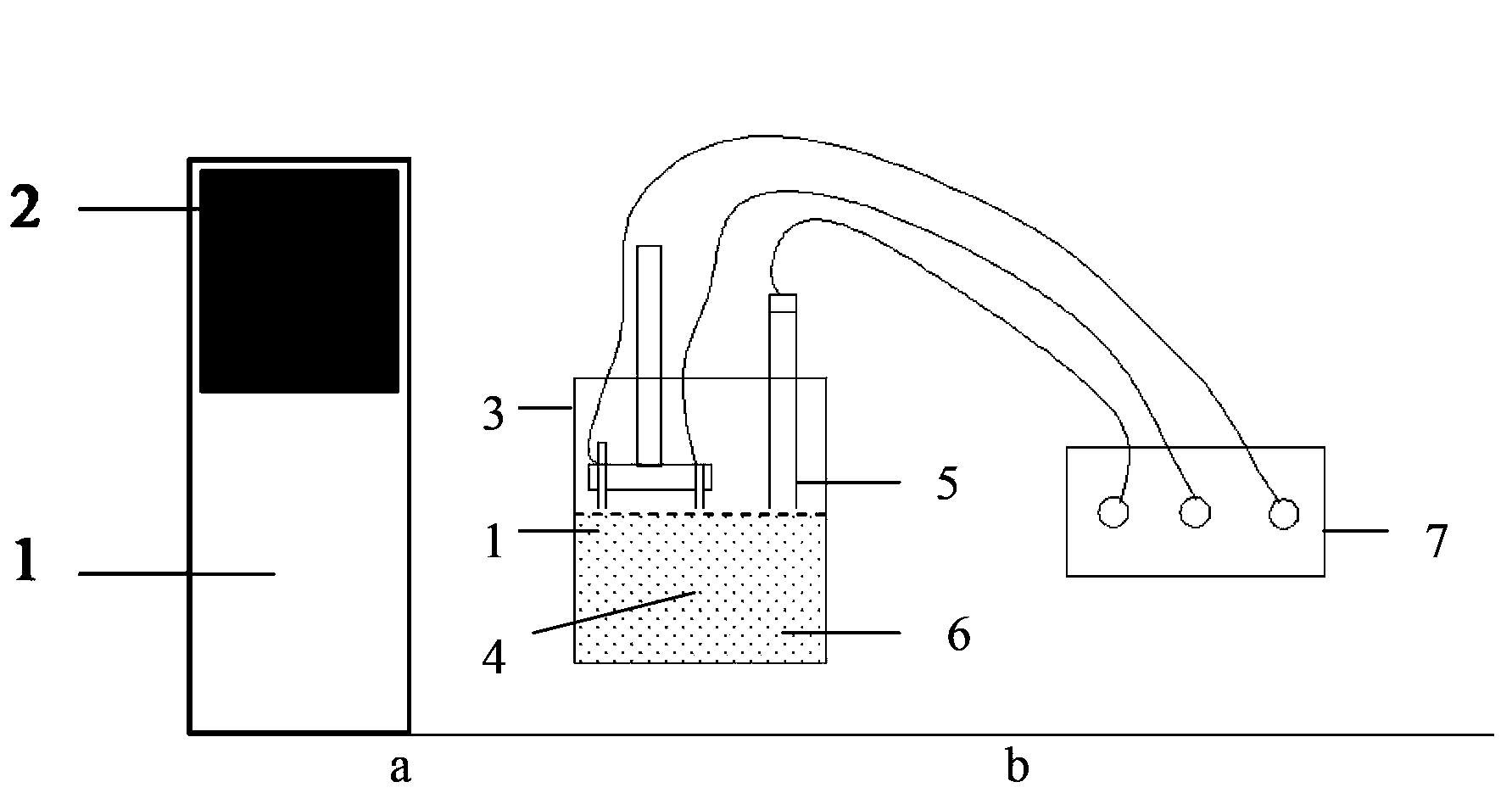
The invention relates to a preparation method for alpha-Fe2O3 photoanode applied to photoelectrolysis. According to the preparation method, a conductive base plate is taken as a working electrode, a Pt metal plate, a graphite plate or the like is taken as a counter electrode, and a Ag / AgCl or saturated calomel electrode is taken as a reference electrode. A three-electrode system formed by enabling an electrodeposition apparatus to communicate with an electrochemical workstation is immersed in a solution of a precursor containing Fe<2+>, a voltage is applied for performing anodization electrodeposition on the surface of electro-conductive glass and further for preparing a FeOOH film, and the film sample is subjected to calcining for obtaining the alpha-Fe2O3 photoanode. The preparation method has the advantages of being cheap in apparatus, simple in apparatus structure, convenient to operate, precisely controllable in film thickness, good in stability and environment-friendly. By employing the method for preparing photoanode during photoelectrolysis, the rapid friendly preparation of the alpha-Fe2O3 photoanode can be realized.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Photoelectrochemical method of separating water into hydrogen and oxygen, using melanins or the analogues, precursors or derivatives thereof as the central electrolysing element
The invention essentially consists in the use of melanins, melanin precursors or melanin derivatives, melanin variants, melanin analogues, natural or synthetic, pure or mixed with organic or inorganic compounds, metals, ions, drugs; as water electrolyzing material, using as sole or main source of energy, natural or synthetic light, coherent or not; in the systems of hydrogen production from water, known as photoelectrochemical systems. These systems integrate as semiconductor material and a water electrolyzer inside a monolithic design, to produce hydrogen directly from water, using light (between 200 to 900 nm) as the main or sole source of energy. At least to basic criteria had to be met: one was that the system or light absorbing compound should generate enough energy to start, lead and complete the photoelectrolysis reaction, being economical, stable and lasting in a water system, requirements met by melanins, representing thus an important and critical advance to solve the central problem of photoelectrochemical designs. The procedure can be applied to generate hydrogen, oxygen and high energy electrons, or the opposite sense, i.e., synthesizing water from the union of hydrogen and oxygen, generating electricity; it can be coupled to other processes, generating a multiplication effect; it can also be used for reduction of carbon dioxide, nitrates and sulphates or others.
Owner:SOLIS HERRERA ARTURO
Photoelectrolysis cells, and related devices and processes
A photoelectrolysis cell is described herein. The cell includes a photoelectrode based on a material having the general formula (Ln1−xMx)(Nb1−yTay)O1+xN2−x. Ln is at least one lanthanide element; M is at least one alkaline earth metal; 0≦x≦0.99; and 0≦y≦1. The photoelectrolysis cell further includes a counter-electrode formed from at least one metal or metallic alloy. An electrolyte which is in contact with both the photoelectrode and the counter-electrode is another component of the cell, along with a means for collecting hydrogen produced by the cell. A related process for producing hydrogen in a photoelectrolysis cell is also described.
Owner:GENERAL ELECTRIC CO
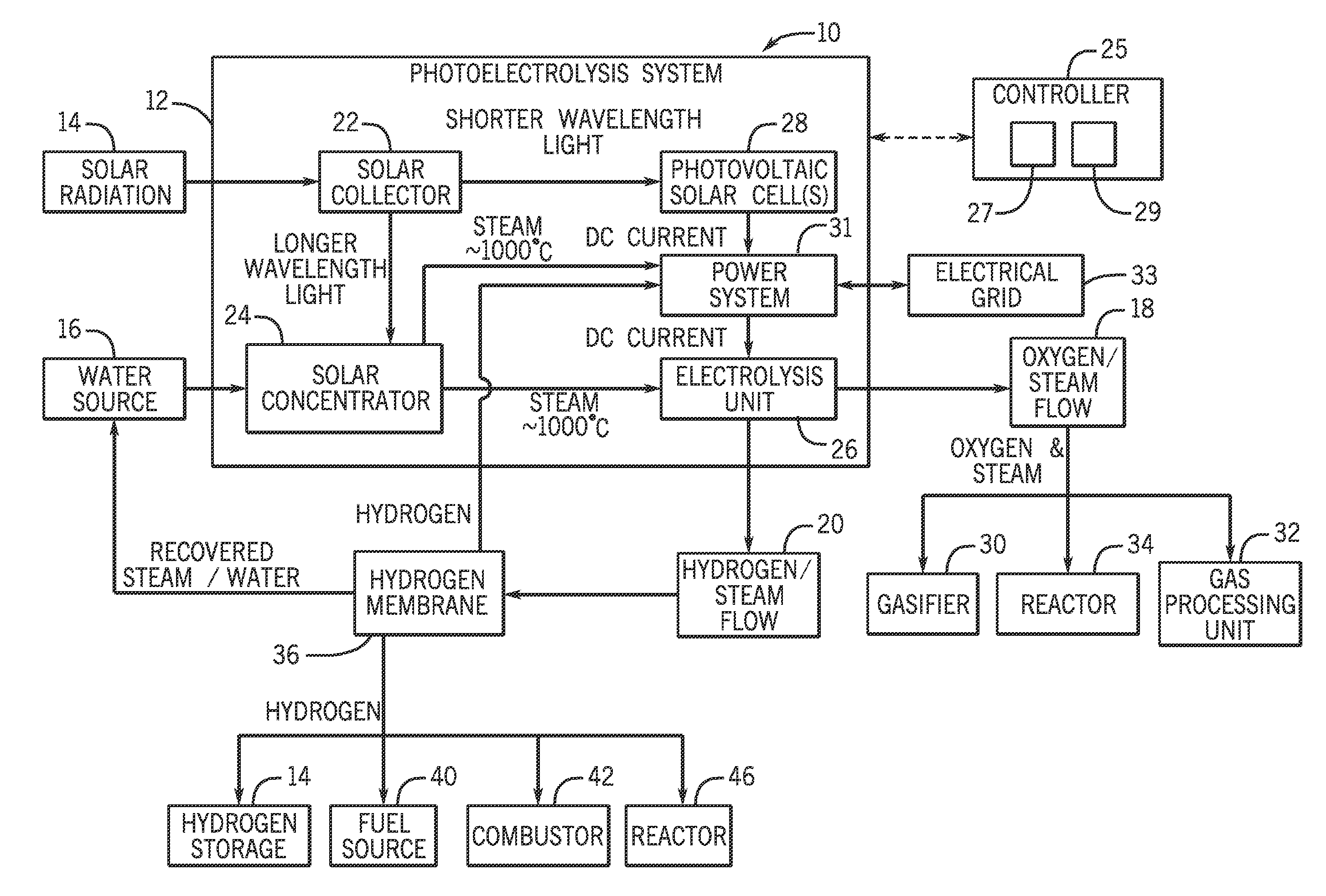
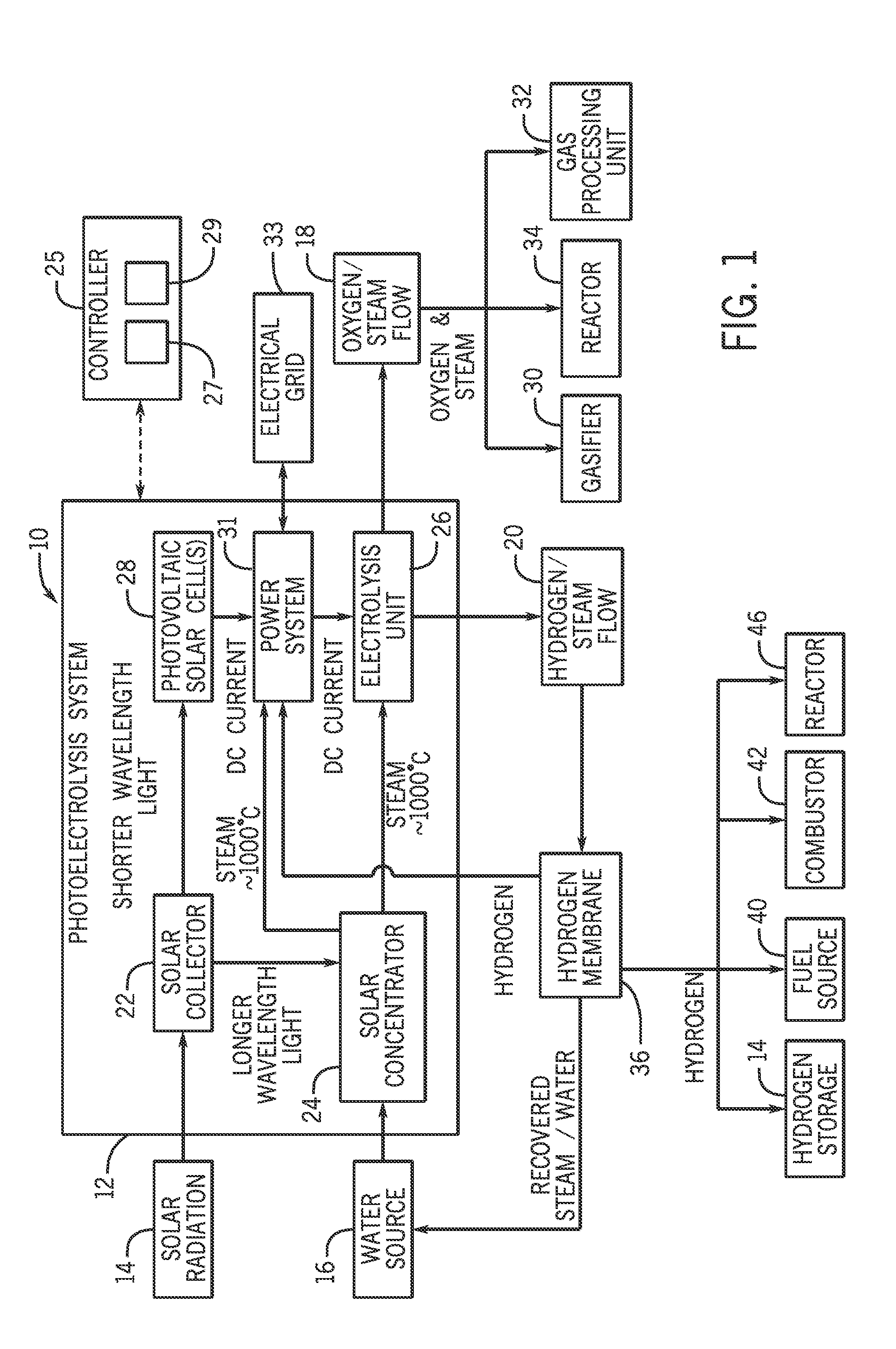
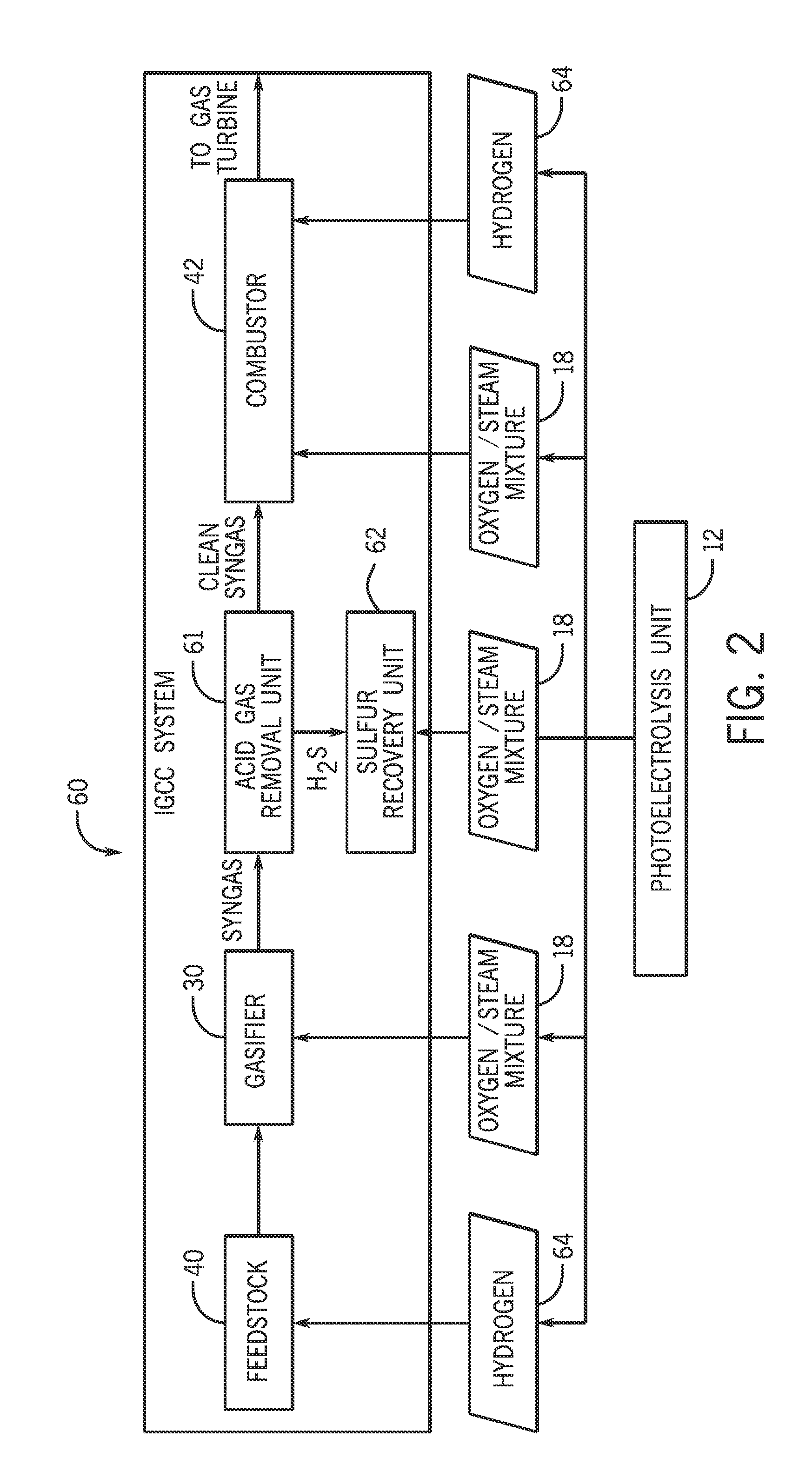
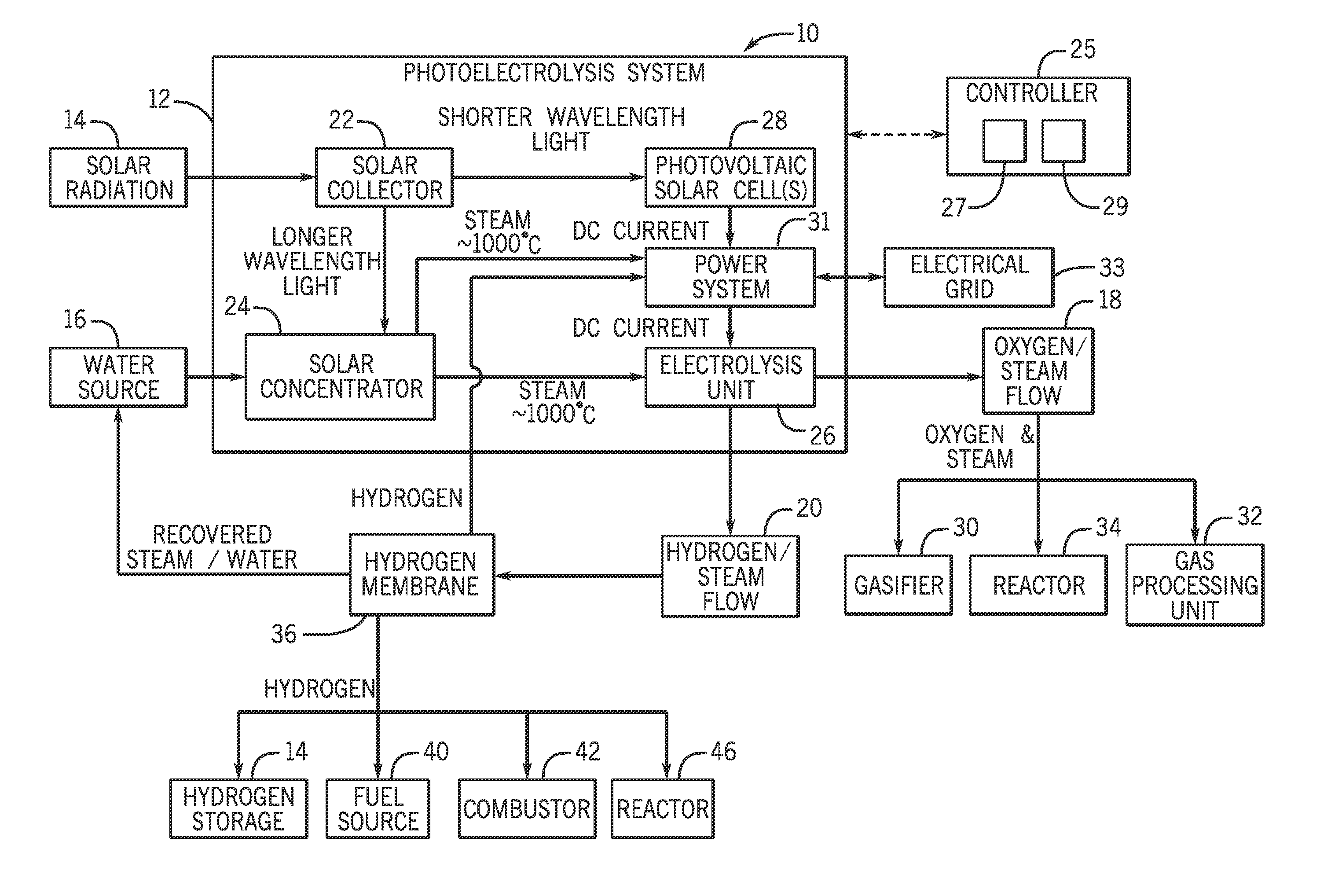
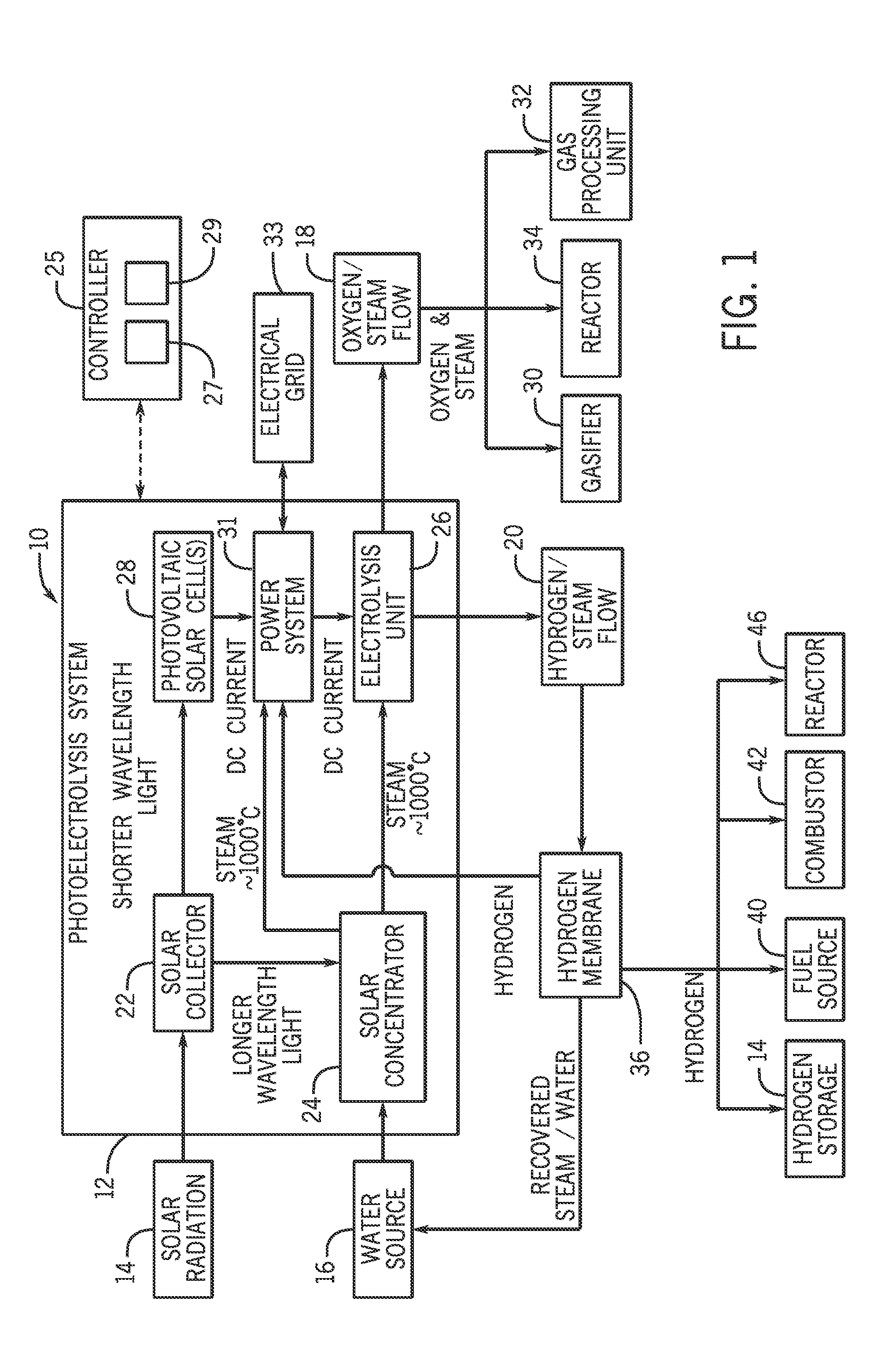
Systems and methods for generating oxygen and hydrogen for plant equipment
A system includes a photoelectrolysis system having a solar collector configured to collect and concentrate solar radiation to heat water, generate electricity, or both. The system also includes an electrolysis unit configured to electrolyze the heated water using at least the generated electricity to produce a first gas mixture and a second gas mixture. The first gas mixture includes oxygen and steam and the second gas mixture includes hydrogen and steam. The system further includes a first device configured to receive and use the first gas mixture as well as a hydrogen membrane configured to receive and separate the hydrogen and steam mixture into a hydrogen component and a steam component.
Owner:AIR PROD & CHEM INC
Photoelectrolysis of water using proton exchange membranes
ActiveUS20050005963A1Enhanced Efficiency StabilityImprove performance stabilityMachining electrodesCellsWater useElectro catalyst
A photoelectrochemical cell which includes a light transmissive enclosure, a semiconductor photoanode disposed within the light transmissive enclosure, a semiconductor photocathode disposed within the light transmissive enclosure, and an electrolytic solution disposed entirely between the semiconductor photoanode and the semiconductor photocathode. This is achieved by the use of semiconductor photoelectrodes (photoanodes and photocathodes) which include a proton exchange membrane having an electrolyte facing surface in contact with the electrolytic solution and a light transmissive wall facing surface, and having a photo electro-catalyst disposed on the light transmissive wall facing surface.
Owner:GAS TECH INST
Method for performing photoelectrolysis of water and preparing hydrogen by using palladium quantum dot modified titanium dioxide nanotube array
InactiveCN102226284AImprove current efficiencyHigh light conversion efficiencyElectrodesTio2 nanotubePhotocathode
The method discloses a method for performing photoelectrolysis of water and preparing hydrogen by using a palladium quantum dot modified titanium dioxide nanotube array, relating to a method for preparing hydrogen. The method utilizes a new titanium dioxide photocathode material to perform the photoelectrocatalysis and electrolysis of water and prepare hydrogen. The method comprises the following steps: the palladium quantum dot modified titanium dioxide nanotube array is used as photocathode, the photoelectrolysis of water is performed in a three-electrode system to prepare hydrogen; the hydrothermal method is adopted to deposit palladium quantum dots on the surface of the TiO2 nanotube array, the particle size is 2.5-4nm, the particles are uniformly distributed on the outer surface of the nanotubes, the two materials are separately used as photoanode and photocathode; and in a three-electrode electrolytic cell, sodium carbonate solution is used as a system and a certain bias is applied to perform the photoelectrolysis of water and prepare hydrogen. Compared with the traditional platinum photocathode, the photocurrent and optical conversion efficiency of the palladium modified titanium dioxide nanotube array photocathode are obviously increased, the hydrogen-producing speed is high, the nanotube array photocathode has good chemical stability and low-cost and large-scale industrial applications of the photocathode can be realized.
Owner:XIAMEN UNIV
Stress-induced bandgap-shifted semiconductor photoelectrolytic/photocatalytic/photovoltaic surface and method for making same
InactiveUS20090110591A1Avoid delaminationImprove matchLight-sensitive devicesInternal combustion piston enginesStress inducedUltraviolet
Owner:NANOPTEK CORP
Stress-induced bandgap-shifted semiconductor photoelectrolytic/photocatalytic/photovoltaic surface and method for making same
ActiveUS20090116095A1Improve efficiencyIncrease photocatalytic surface areaElectrolytic capacitorsSemiconductor/solid-state device manufacturingStress inducedUltraviolet
Titania is a semiconductor and photocatalyst that is also chemically inert. With its bandgap of 3.0, to activate the photocatalytic property of titania requires light of about 390 nm wavelength, which is in the ultra-violet, where sunlight is very low in intensity. A method and devices are disclosed wherein stress is induced and managed in a thin film of titania in order to shift and lower the bandgap energy into the longer wavelengths that are more abundant in sunlight. Applications of this stress-induced bandgap-shifted titania photocatalytic surface include photoelectrolysis for production of hydrogen gas from water, photovoltaics for production of electricity, and photocatalysis for detoxification and disinfection.
Owner:NANOPTEK CORP
Preparation method for electro-oxidation synthesis of one-dimensional nano-oxide structure
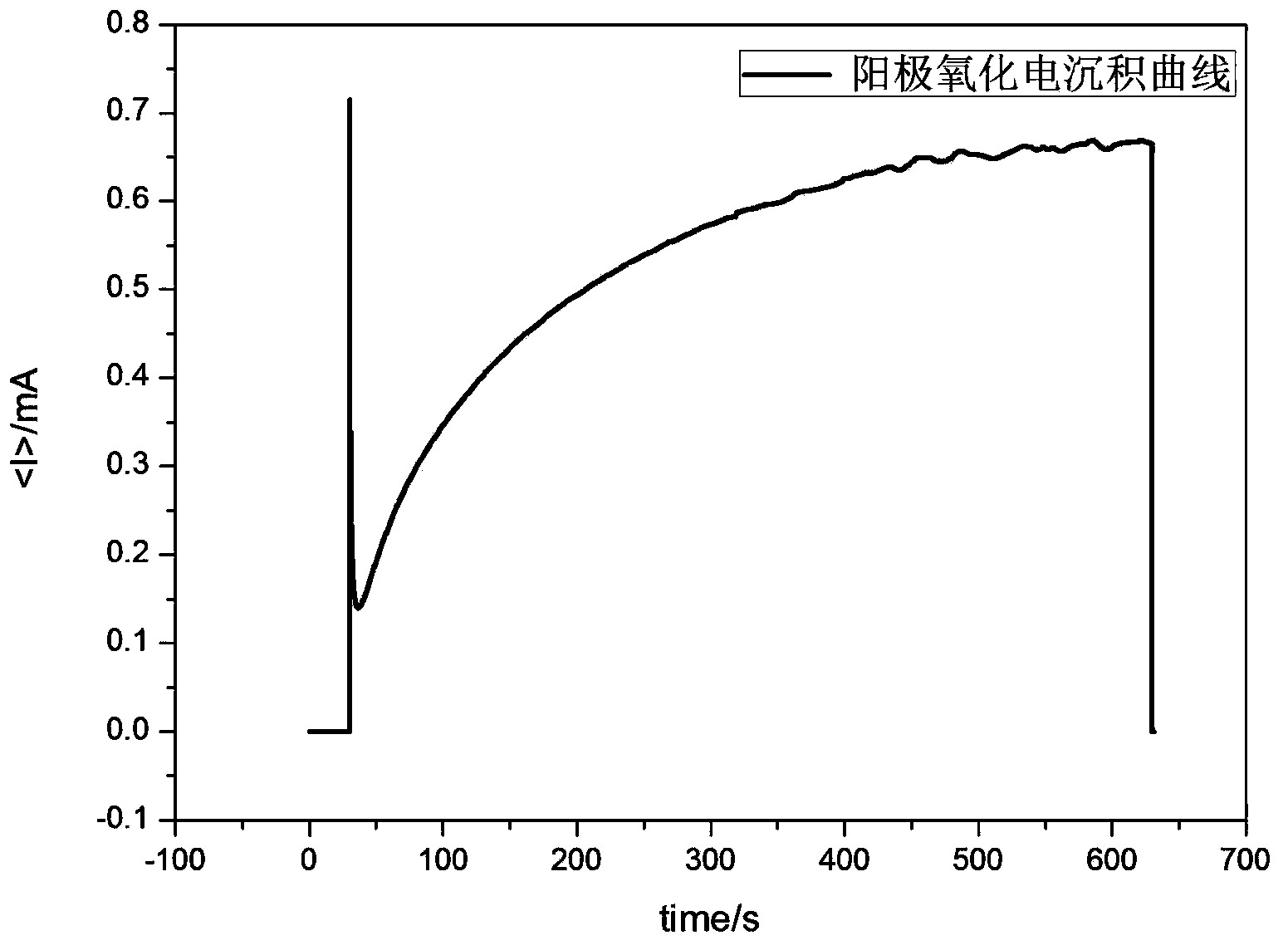
The invention relates to a preparation method for one-dimensional nano-oxides in the field of photoelectrocatalysis. An electrochemical system is built with a Fe2+ precursor solution as an electrolyte solution, deionized water and polyols of different proportions as solvents, a conductive substrate as a working electrode, a Pt metal plate or a graphite plate as a counter electrode, and Ag / AgCl or a saturated calomel electrode as a reference electrode. A FeOOH thin film is prepared on a surface of conductive glass by means of electrodeposition; the thin film sample is dipped into a shape protective agent and then calcined into an alpha-Fe2O3 photoanode. Using such a method to prepare the photoanode, tight integration of the alpha-Fe2O3 photoanode thin film and the conductive substrate can be realized and the stability of the photoanode during photoelectrolysis is improved. Using the photoanode prepared by means of such a method during photoelectrolysis, accurate control over the thickness of the alpha-Fe2O3 photoanode thin film can be realized; thin films different in thickness can be deposited under different electric quantities by controlling the time of the current, thereby providing important basis for discussing the transfer mechanism of current carriers of a photoelectrolytic tank and the relation of optical characteristics to film thicknesses.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Photoelectrochemical method of separating water into hydrogen and oxygen, using melanins or the analogues, precursors or derivatives thereof as the central electrolysing element
The invention essentially consists in the use of melanins, melanin precursors or melanin derivatives, melanin variants, melanin analogues, natural or synthetic, pure or mixed with organic or inorganic compounds, metals, ions, drugs; as water electrolyzing material, using as sole or main source of energy, natural or synthetic light, coherent or not; in the systems of hydrogen production from water, known as photoelectrochemical systems. These systems integrate as semiconductor material and a water electrolyzer inside a monolithic design, to produce hydrogen directly from water, using light (between 200 to 900 nm) as the main or sole source of energy. At least to basic criteria had to be met: one was that the system or light absorbing compound should generate enough energy to start, lead and complete the photoelectrolysis reaction, being economical, stable and lasting in a water system, requirements met by melanins, representing thus an important and critical advance to solve the central problem of photoelectrochemical designs. The procedure can be applied to generate hydrogen, oxygen and high energy electrons, or the opposite sense, i.e., synthesizing water from the union of hydrogen and oxygen, generating electricity; it can be coupled to other processes, generating a multiplication effect; it can also be used for reduction of carbon dioxide, nitrates and sulphates or others.
Owner:SOLIS HERRERA ARTURO
Stress-induced bandgap-shifted semiconductor photoelectrolytic/photocatalytic/photovoltaic surface and method for making same
InactiveUS7995871B2Avoid delaminationImprove matchElectrolytic capacitorsSemiconductor/solid-state device manufacturingStress inducedElectricity
Titania is a semiconductor and photocatalyst that is also chemically inert. With its bandgap of 3.0, to activate the photocatalytic property of titania requires light of about 390 nm wavelength, which is in the ultra-violet, where sunlight is very low in intensity. A method and devices are disclosed wherein stress is induced and managed in a thin film of titania in order to shift and lower the bandgap energy into the longer wavelengths that are more abundant in sunlight. Applications of this stress-induced bandgap-shifted titania photocatalytic surface include photoelectrolysis for production of hydrogen gas from water, photovoltaics for production of electricity, and photocatalysis for detoxification and disinfection.
Owner:NANOPTEK CORP
Bandgap-shifted semiconductor surface and method for making same, and apparatus for using same
ActiveUS20150214411A1Improve efficiencyIncrease photocatalytic surface areaCellsPhotovoltaicsUltravioletMaterials science
Titania is a semiconductor and photocatalyst that is also chemically inert. With its bandgap of 3.2 and greater, to activate the photocatalytic property of titania requires light of about 390 nm wavelength, which is in the ultra-violet, where sunlight is very low in intensity. A method and devices are disclosed wherein stress is induced and managed in a thin film of titania in order to shift and lower the bandgap energy into the longer wavelengths that are more abundant in sunlight. Applications of this stress-induced bandgap-shifted titania photocatalytic surface include photoelectrolysis for production of hydrogen gas from water, photovoltaics for production of electricity, and photocatalysis for detoxification and disinfection.
Owner:NANOPTEK CORP
Bandgap-shifted semiconductor surface and method for making same, and apparatus for using same
ActiveUS20080299697A1Improve efficiencyIncrease photocatalytic surface areaPhotovoltaic supportsElectrolysis componentsPhoto catalysisSemiconductor
Titania is a semiconductor and photocatalyst that is also chemically inert. With its bandgap of 3.2 and greater, to activate the photocatalytic property of titania requires light of about 390 nm wavelength, which is in the ultra-violet, where sunlight is very low in intensity. A method and devices are disclosed wherein stress is induced and managed in a thin film of titania in order to shift and lower the bandgap energy into the longer wavelengths that are more abundant in sunlight. Applications of this stress-induced bandgap-shifted titania photocatalytic surface include photoelectrolysis for production of hydrogen gas from water, photovoltaics for production of electricity, and photocatalysis for detoxification and disinfection.
Owner:NANOPTEK CORP
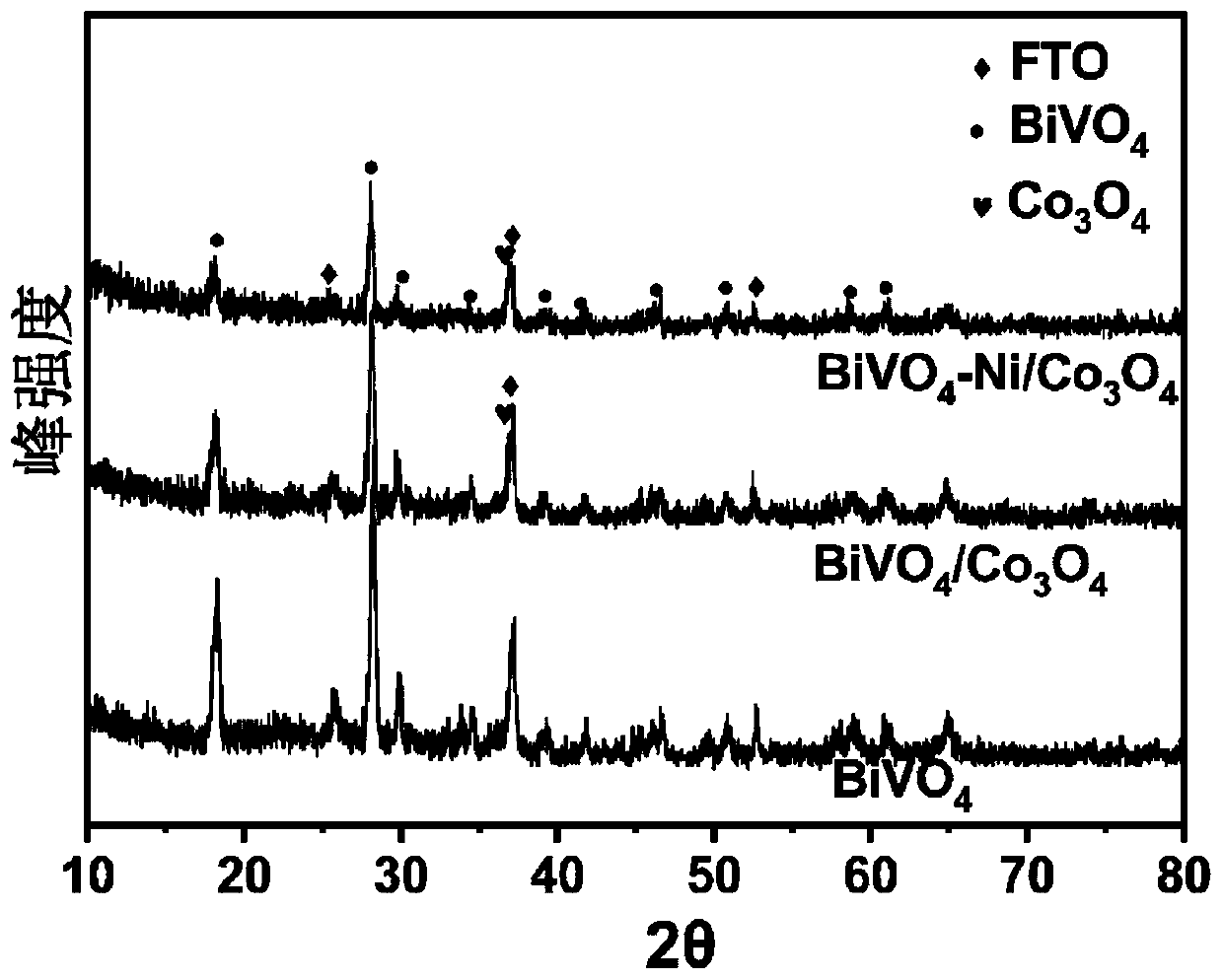
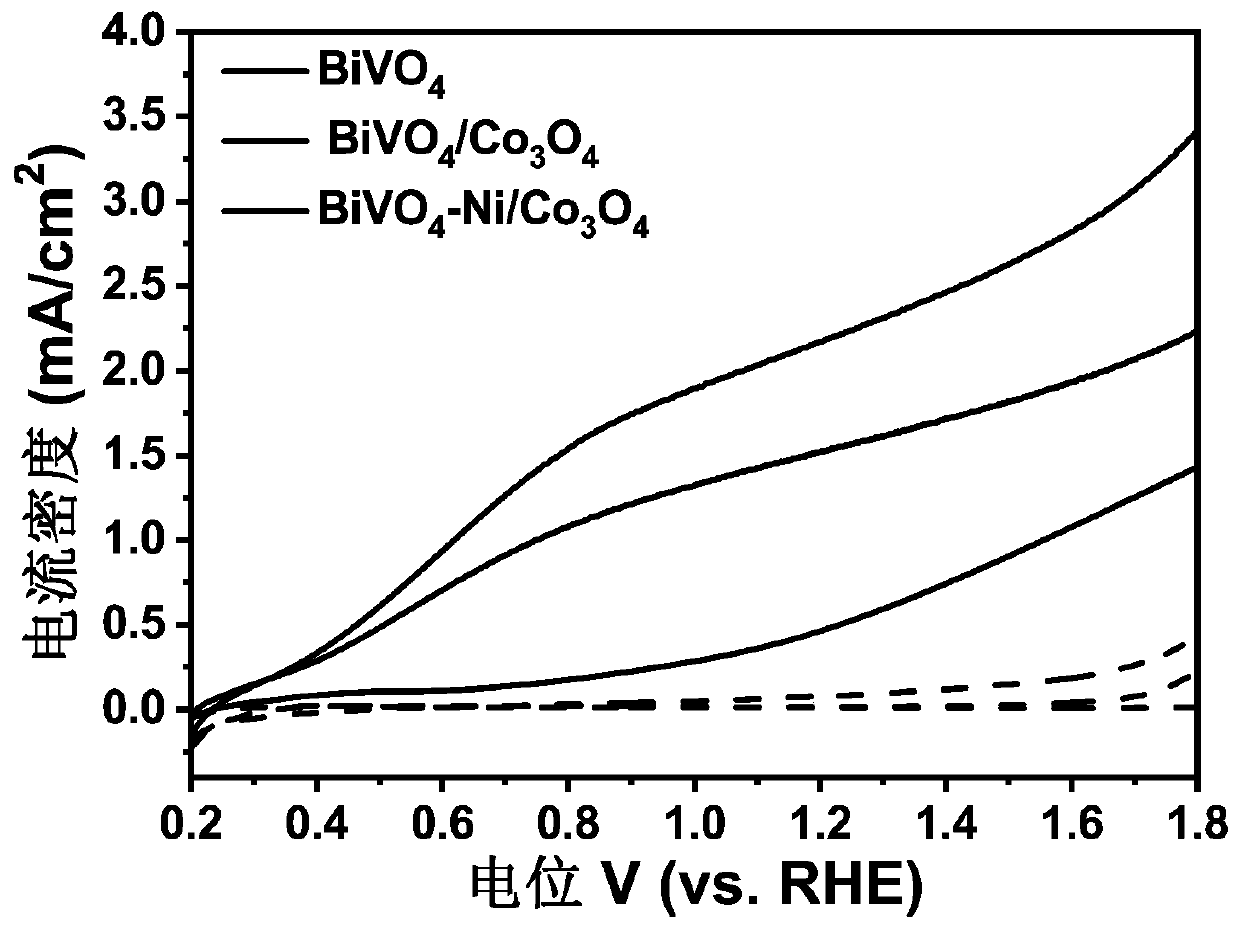
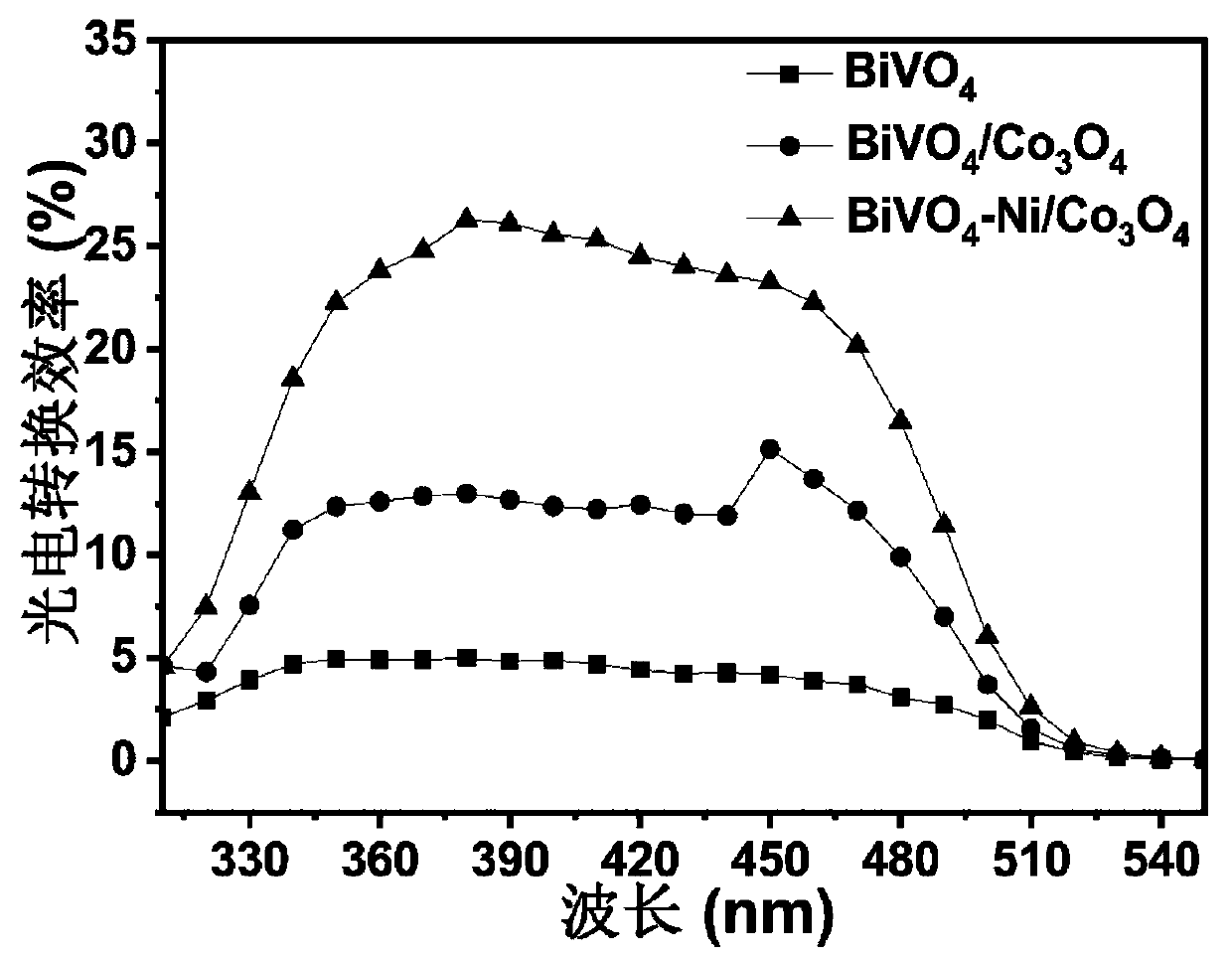
Synthesis method of BiVO4-Ni/Co3O4 heterojunction and application of BiVO4-Ni/Co3O4 heterojunction to photoelectrochemical hydrolysis
InactiveCN111569896AImprove photoelectrochemical performanceLow costEnergy inputMetal/metal-oxides/metal-hydroxide catalystsHeterojunctionInterfacial reaction
The invention belongs to the technical field of nano composite materials and relates to a synthesis method of a BiVO4-Ni / Co3O4 heterojunction. The synthesis method comprises the following steps of: growing a layer of BiOI nanoparticles on an FTO substrate by adopting an electro-deposition method; dropwise adding a vanadyl acetylacetonate aqueous solution on the surface of the FTO; performing calcining at a high temperature to generate bismuth vanadate (BiVO4); obliquely placing FTO in a deionized water solution containing Co (NO3) 2.6 H2O, Ni (NO3) 2.6 H2O, C6H12N4, CH4N2O and NH4F through continuous ion adsorption reaction; performing hydrothermal reaction at 120-200 DEG C for 2-5 hours, taking out an obtained product, cleaning the product with deionized water; annealing the product at 300-500 DEG C for 1.5-3 hours; and naturally cooling the product to room temperature to obtain the BiVO4-Ni / Co3O4 heterojunction. The prepared heterojunction is used as a photoelectrode to be applied tophotoelectrochemical hydrolysis reaction. According to the preparation method, a simple electro-deposition method and a hydrothermal method are utilized, therefore, the method is simple in operationand has good repeatability; the used materials are low in cost, large in reserves and non-toxic, and meets the requirement for environmental friendliness; the prepared material can significantly reduce the interface reaction barrier, effectively inhibit solid-liquid interface charge recombination, accelerate water oxidation reaction kinetics and improve the photocurrent density so as to better utilize solar energy.
Owner:JIANGSU UNIV
Stress-induced bandgap-shifted semiconductor photoelectrolytic/photocatalytic/photovoltaic surface and method for making same
InactiveUS20090101420A1Avoid delaminationImprove matchInternal combustion piston enginesDigital data processing detailsElectricityHydrogen
Owner:NANOPTEK CORP
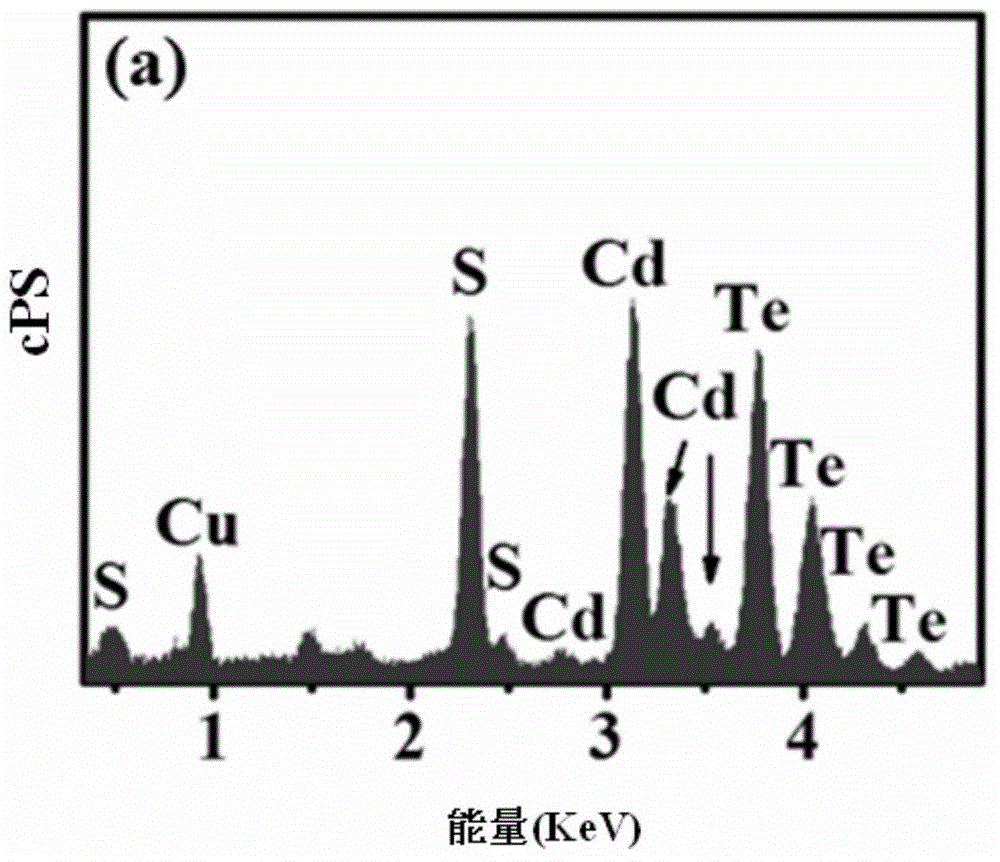
Gourd string structure cadmium sulfide-tellurium heterojunction photoelectrolysis composite material, preparation method and use
ActiveCN105016313AExcellent hydrogen production rateGreat application potentialMaterial nanotechnologyCadmium sulfidesHeterojunctionMaterials preparation
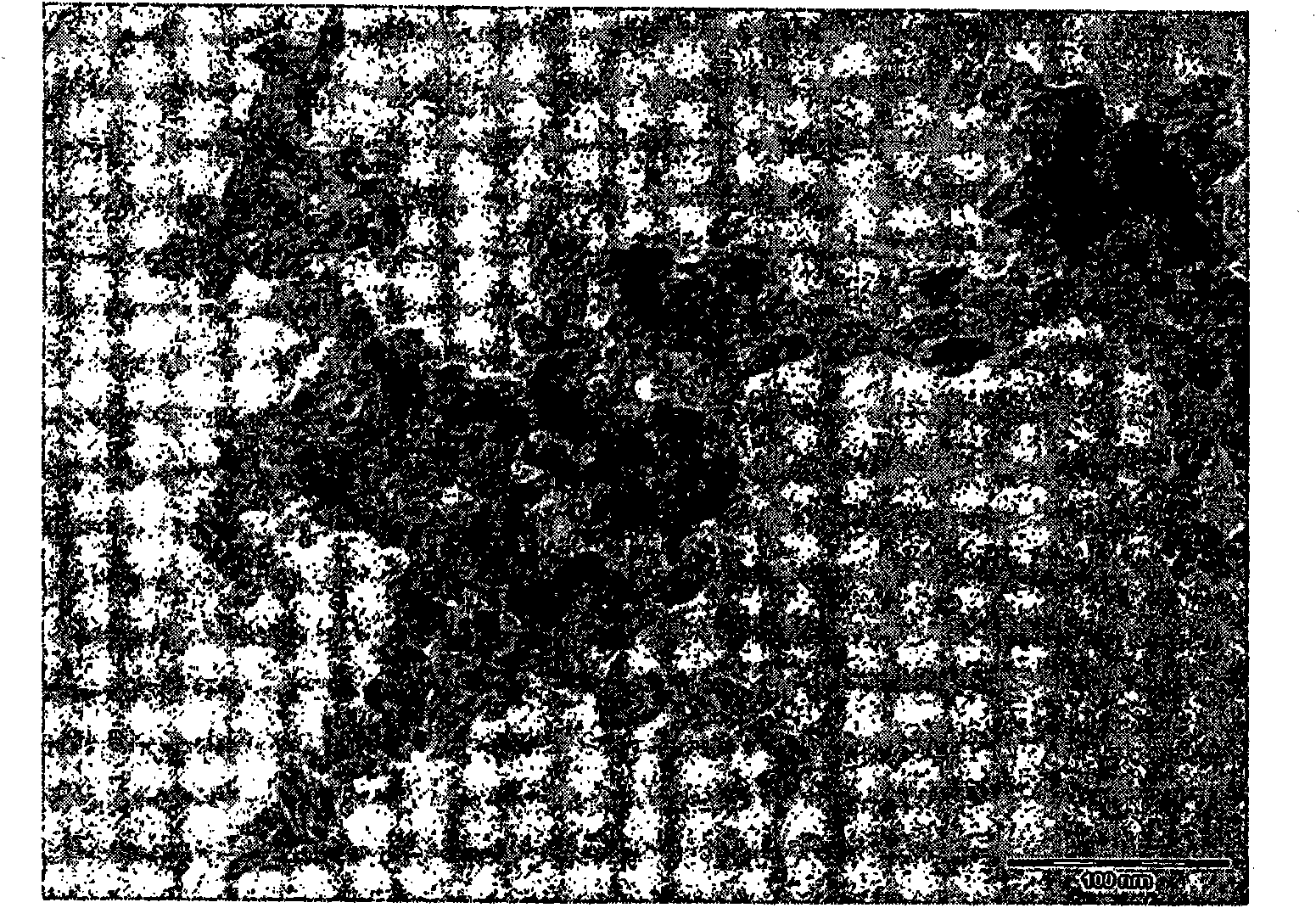
The present invention relates to a gourd string structure cadmium sulfide-tellurium heterojunction photoelectrolysis composite material preparation method. The method comprises the following steps: S1: adding a precursor containing a sulfur telluride source and a a precursor containing a cadmium source to an organic solvent, and sufficiently stirring and uniformly mixing the substances to acquire a precursor reaction liquid; S2: performing thermostatical reacting on the precursor reaction liquid by means of two-stage microwave heating to acquire the gourd string structure cadmium sulfide-tellurium heterojunction photoelectrolysis composite material. According to the preparation method, the gourd string structure cadmium sulfide-tellurium heterojunction photoelectrolysis composite material with an excellent hydrogen production property is acquired by the selection and combination of specific process steps and process parameters. The composite material can be used in the field of water photodecomposition of hydrogen production, and has good application prospects and industrialization potential.
Owner:WENZHOU UNIVERSITY
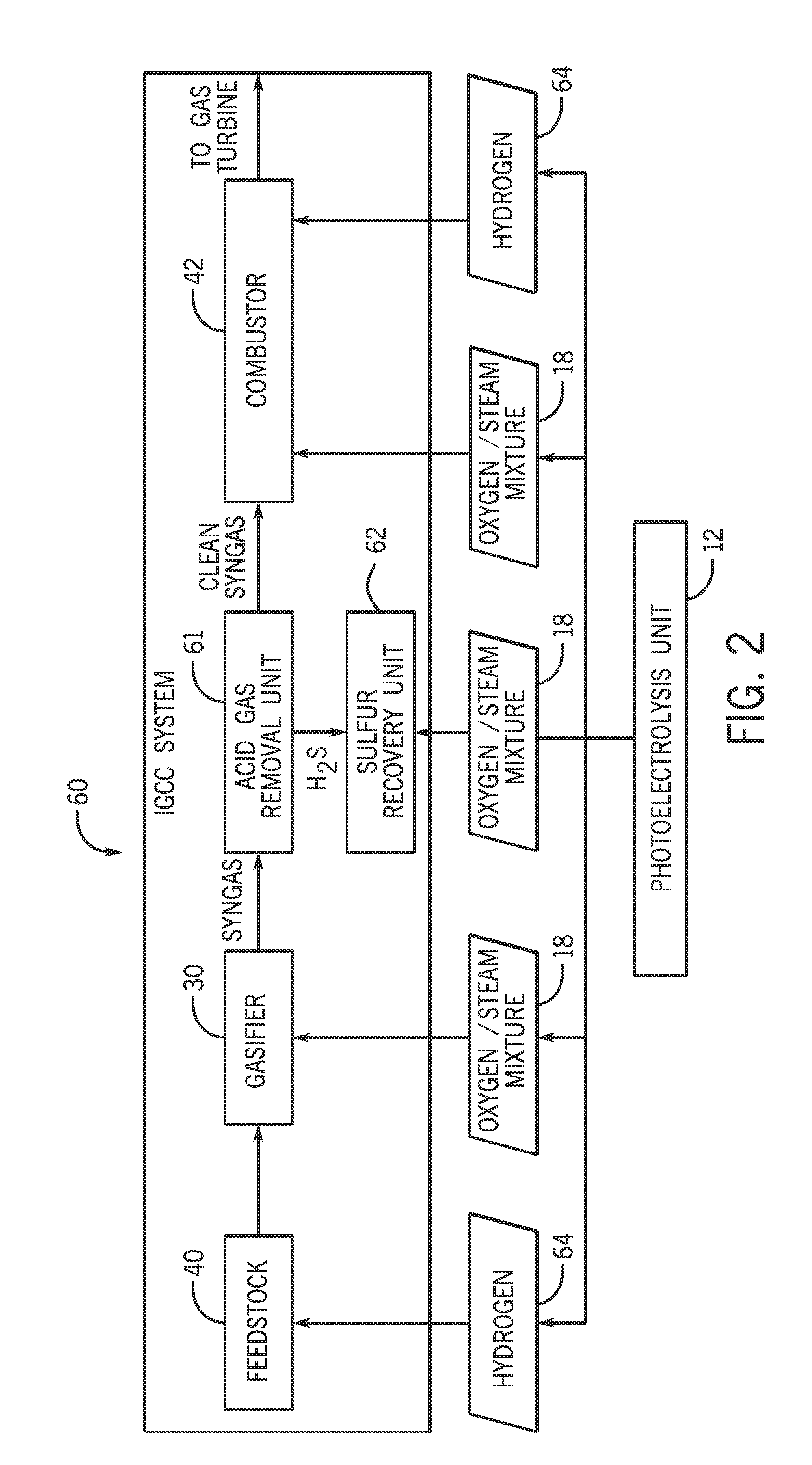
Production of gasoline from fermentable feedstocks
ActiveUS8241881B2Easy to separateImprove isolationElectrolysis componentsBacteriaCatalytic reformingBiodiesel
Compositions and methods for forming hexane, and, optionally, gasoline and / or components of a gasoline composition, from fermentable sugars are disclosed. The sugars are fermented using a bacteria or yeast that predominantly forms butyric acid. The butyric acid is subjected to Kolbe or photo-Kolbe electrolysis to form hexane. The hexane can be subjected to catalytic, reforming and / or isomerization steps to form higher octane products, which are or can be included in gasoline compositions. In one aspect, the fermentable sugars are derived from lignocellulosic materials such as wood products, switchgrass, or agricultural wastes. These materials are delignified to form lignin, cellulose and hemicellulose. The cellulose and hemicellulose are depolymerized to form glycose and xylose, either or both of which can be fermented by the bacteria. The lignin can be used to generate heat energy and / or electric energy for use in one or more process steps, such as the fermentation, product isolation, Kolbe electrolysis, catalytic reforming and / or isomerization steps. Alternatively, the lignin can be converted to synthesis gas, which can then be subjected to Fischer-Tropsch synthesis, or converted to methanol and / or ethanol. Thus, the methods described herein can convert biomass to a fuel composition or fuel additive, which can be used in a conventional gasoline engine, unlike traditional fuels such as ethanol or biodiesel.
Owner:CPS BIOFUELS INC
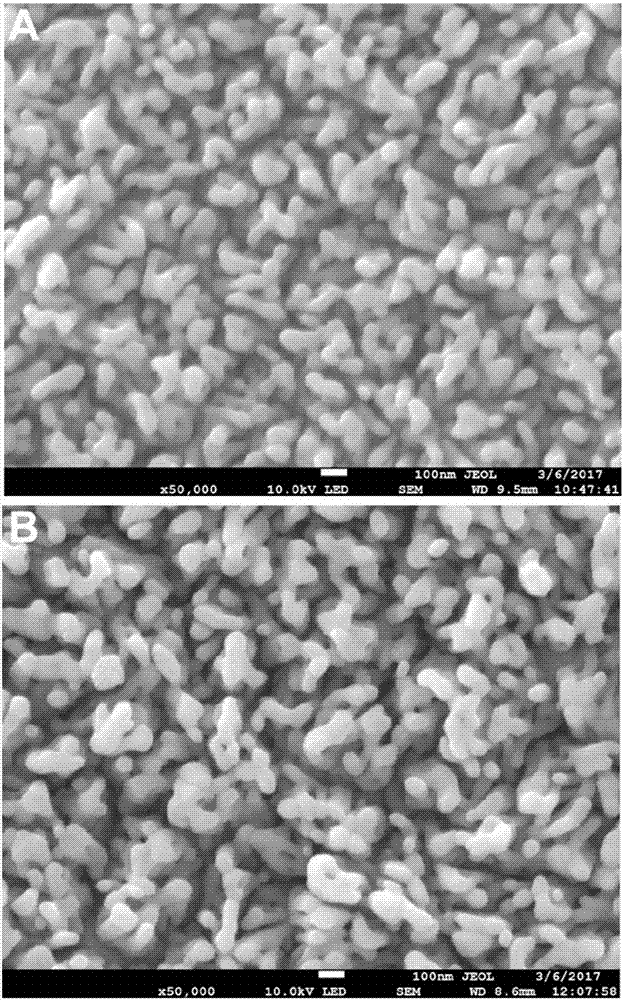
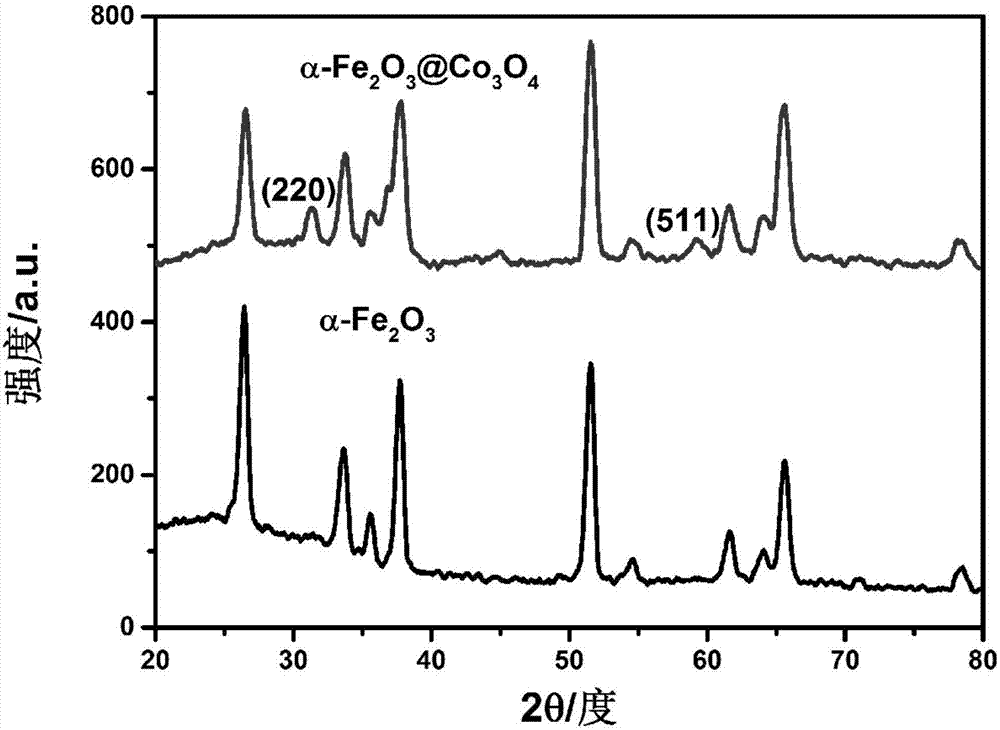
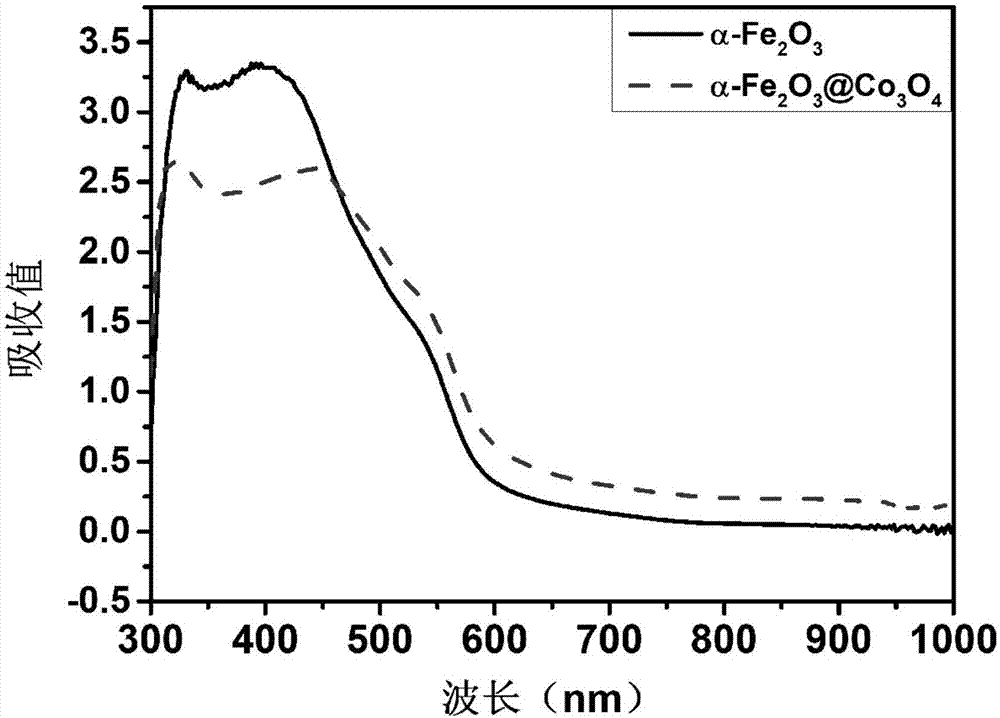
Cobaltosic oxide composite alpha type iron oxide vermicule nanostructure array photoanode and preparation method and application thereof
ActiveCN107268024ATake full advantage of the high specific surface areaGood oxygen evolution catalytic activityMaterial nanotechnologyElectrodesElectron holeNew energy
The invention relates to a cobaltosic oxide composite alpha type iron oxide vermicule nanostructure array photoanode and a preparation method and application thereof, and belongs to the technical field of new energy materials. Through hydrothermal reaction, alpha type iron oxide grows on an electric conducting substrate in a manner of vermicule nanostructure array; cobaltosic oxide nanoparticles are continuously attached on the alpha type iron oxide by using a hydrothermal method; and high specific surface area and excellent oxygen evolution catalysis activity of the cobaltosic oxide nanoparticles are fully used for effectively inhibiting combination of electron-hole pairs, so that the performances of photoelectrolysis water are greatly improved. Meanwhile, the hydrothermal process suitable for industrialization is adopted; in the process of preparing a cobaltosic oxide modified alpha type iron oxide photoanode, used materials are low in cost, so that the production cost is reduced, and large-scale production is realized.
Owner:SOUTHWEST UNIVERSITY
Process for the preparation of titanium dioxide with nanometric dimensions and controlled shape
InactiveUS20100316561A1Simple and economical methodHighly replicable resultMaterial nanotechnologyNanostructure manufactureHydrogenTandem cell
The present invention relates to an industrial applicable process for the preparation of materials with nanometric dimensions and controlled shape, based on titanium dioxide. The invention also relates to a process for the preparation of titanium dioxide nanorods with anatase phase composition, which are highly suitable for applications involving photovoltaic cells, particularly Dye Sensitized Solar Cells (DSSC), photoelectrolysis cells and tandem cells for the conversion of solar energy and the production of hydrogen.
Owner:DAUNIA SOLAR CELL
Method of photoelectrolysis
InactiveUS20070017794A1Improve efficiencyCost effective productionPhotography auxillary processesElectrolysis componentsPhysical chemistryIon-exchange membranes
A method for the photoelectrolysis of a liquid or gaseous species, comprises irradiating an ion exchange membrane of a membrane electrode assembly, wherein the membrane is an optically transparent material and comprises the species.
Owner:ITM POWER LTD FORMELY KNOWN AS STAMFORD MEMORY POLYMERS LTD (GB)
Process for the preparation of titanium dioxide with nanometric dimensions and controlled shape
InactiveCN101952202AEasy to achieve industrial scaleLow production costMaterial nanotechnologyNanostructure manufactureTandem cellPhase composition
The present invention relates to an industrial applicable process for the preparation of materials with nanometric dimensions and controlled shape, based on titanium dioxide. The invention also relates to a process for the preparation of titanium dioxide nanorods with anatase phase composition, which are highly suitable for applications involving photovoltaic cells, particularly Dye Sensitized Solar Cells (DSSC), photoelectrolysis cells and tandem cells for the conversion of solar energy and the production of hydrogen.
Owner:DAONIA SOLAR CELLS CO LTD
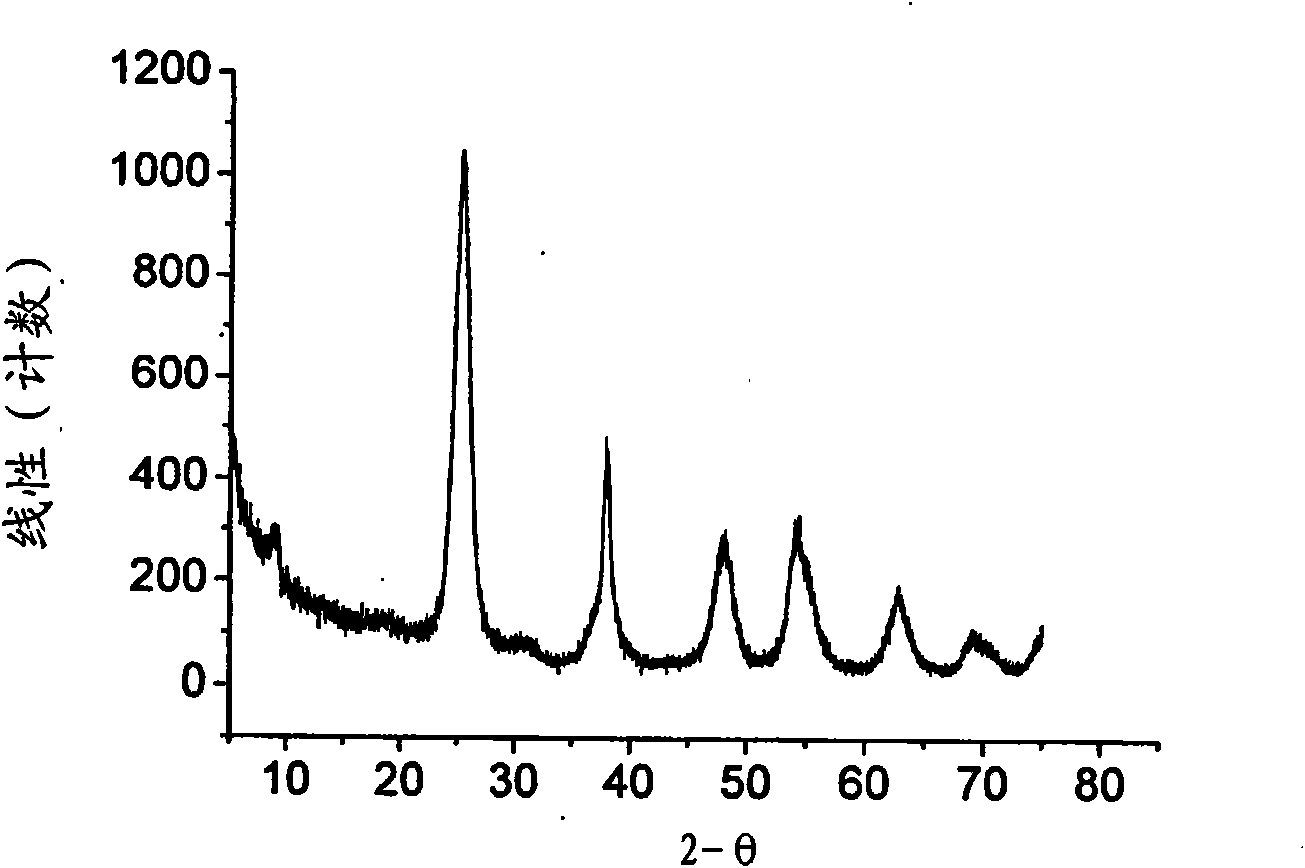
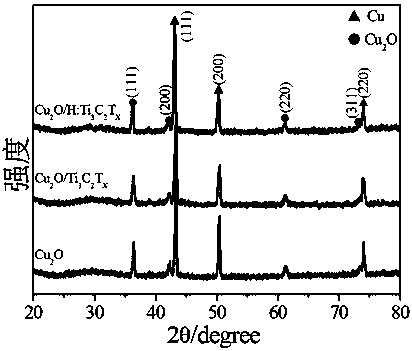
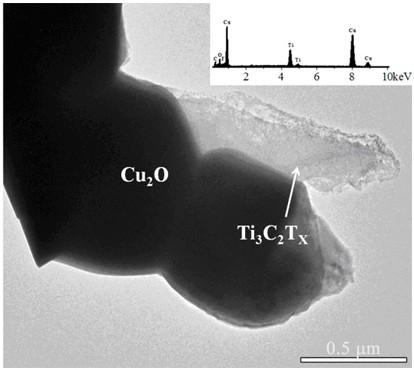
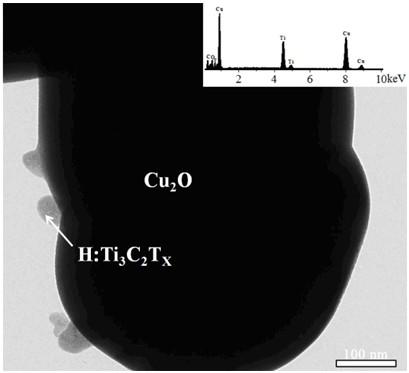
Hydrogen-reduced thin-layer titanium carbide loaded cuprous oxide photo-cathode material for photoelectrolysis of water and preparation method for photo-cathode material
ActiveCN109706478AImprove photoelectrochemical performanceSimple preparation processEnergy inputElectrodesDecompositionThin layer
The invention discloses a hydrogen-reduced thin-layer titanium carbide loaded cuprous oxide photo-cathode material for photoelectrolysis of water and a preparation method for the photo-cathode material. According to the method, the composite photo-electrode material with relatively high photoelectrochemical properties, i.e., hydrogen-reduced thin-layer titanium carbide loaded cuprous oxide, i.e.,Cu2O / H:Ti3C2Tx is synthesized through preparing cuprous oxide by taking conductive glass or copper as a substrate and loading the surface of the cuprous oxide with hydrogen-reduced thin-layer titaniumcarbide. The electrode material comprises a substrate, cuprous oxide and the hydrogen-reduced thin-layer titanium carbide. The hydrogen-reduced thin-layer titanium carbide is loaded with a cuprous oxide electrode, so that the photoelectrochemical properties of the electrode material can be remarkably improved, and the electrode material can be applied to the fields of preparation of solar photovoltaic cells, construction of photoelectrochemical sensors, photoelectro-catalyzed water-decomposition hydrogen production and the like.
Owner:XIANGTAN UNIV
Systems and methods for generating oxygen and hydrogen for plant equipment
A system includes a photoelectrolysis system having a solar collector configured to collect and concentrate solar radiation to heat water, generate electricity, or both. The system also includes an electrolysis unit configured to electrolyze the heated water using at least the generated electricity to produce a first gas mixture and a second gas mixture. The first gas mixture includes oxygen and steam and the second gas mixture includes hydrogen and steam. The system further includes a first device configured to receive and use the first gas mixture as well as a hydrogen membrane configured to receive and separate the hydrogen and steam mixture into a hydrogen component and a steam component.
Owner:AIR PROD & CHEM INC
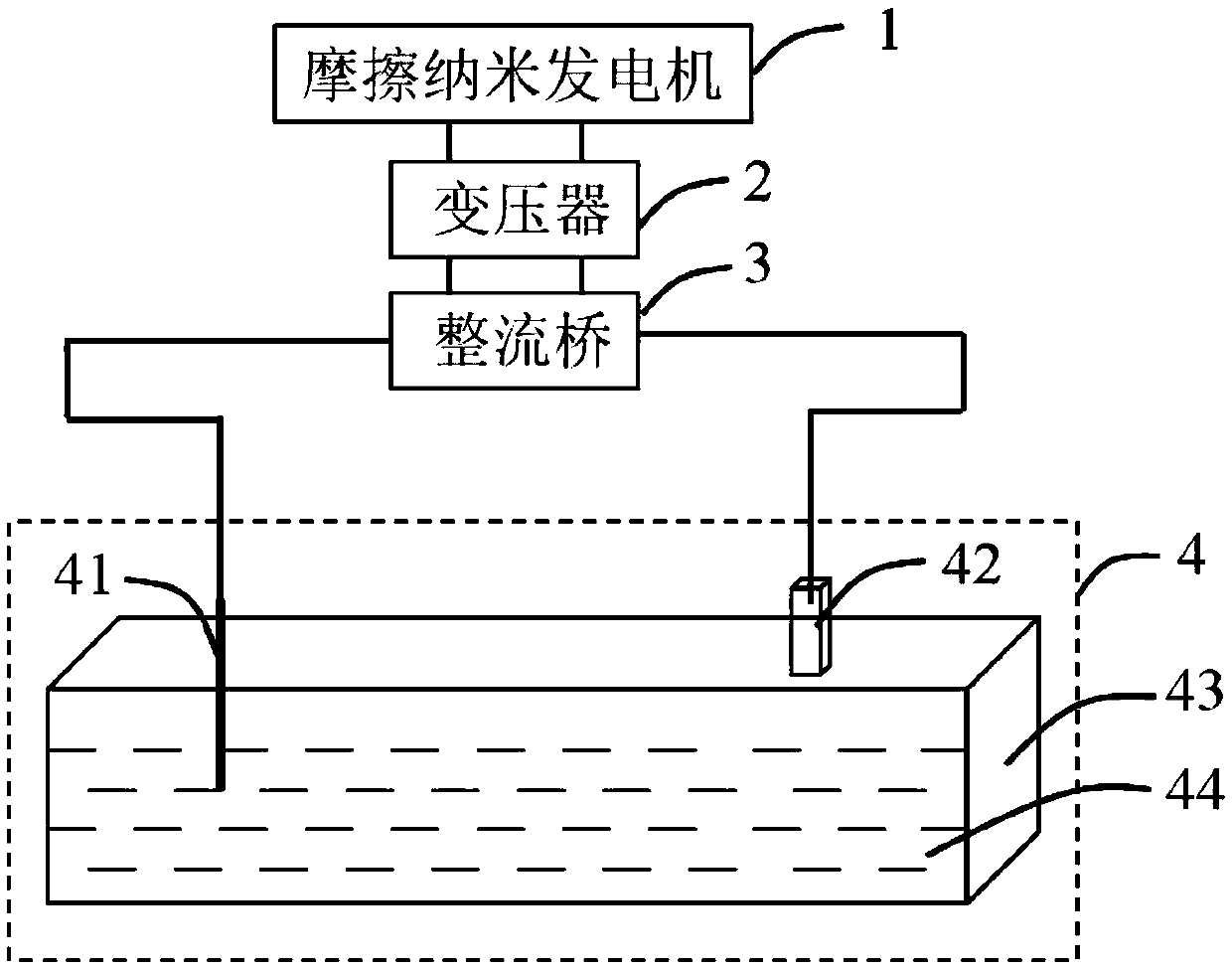
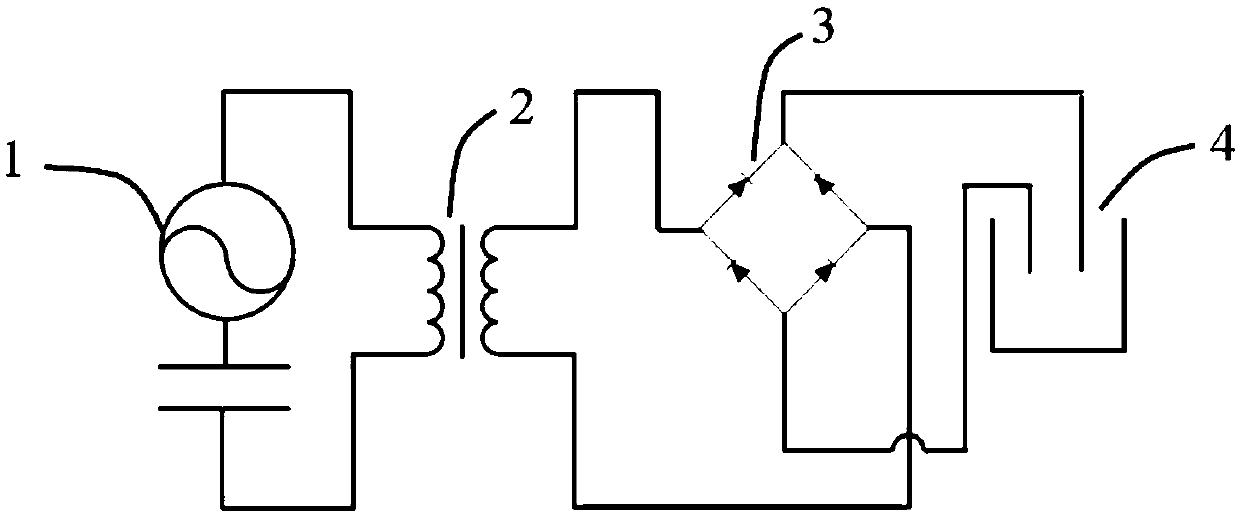
Self-driven water photoelectrolysis system based on friction nano power generator
InactiveCN108675385AMake up for the defect of poor conductivityImprove hydrogen production efficiencyBatteries circuit arrangementsWater/sewage treatment by irradiationLow voltageTransformer
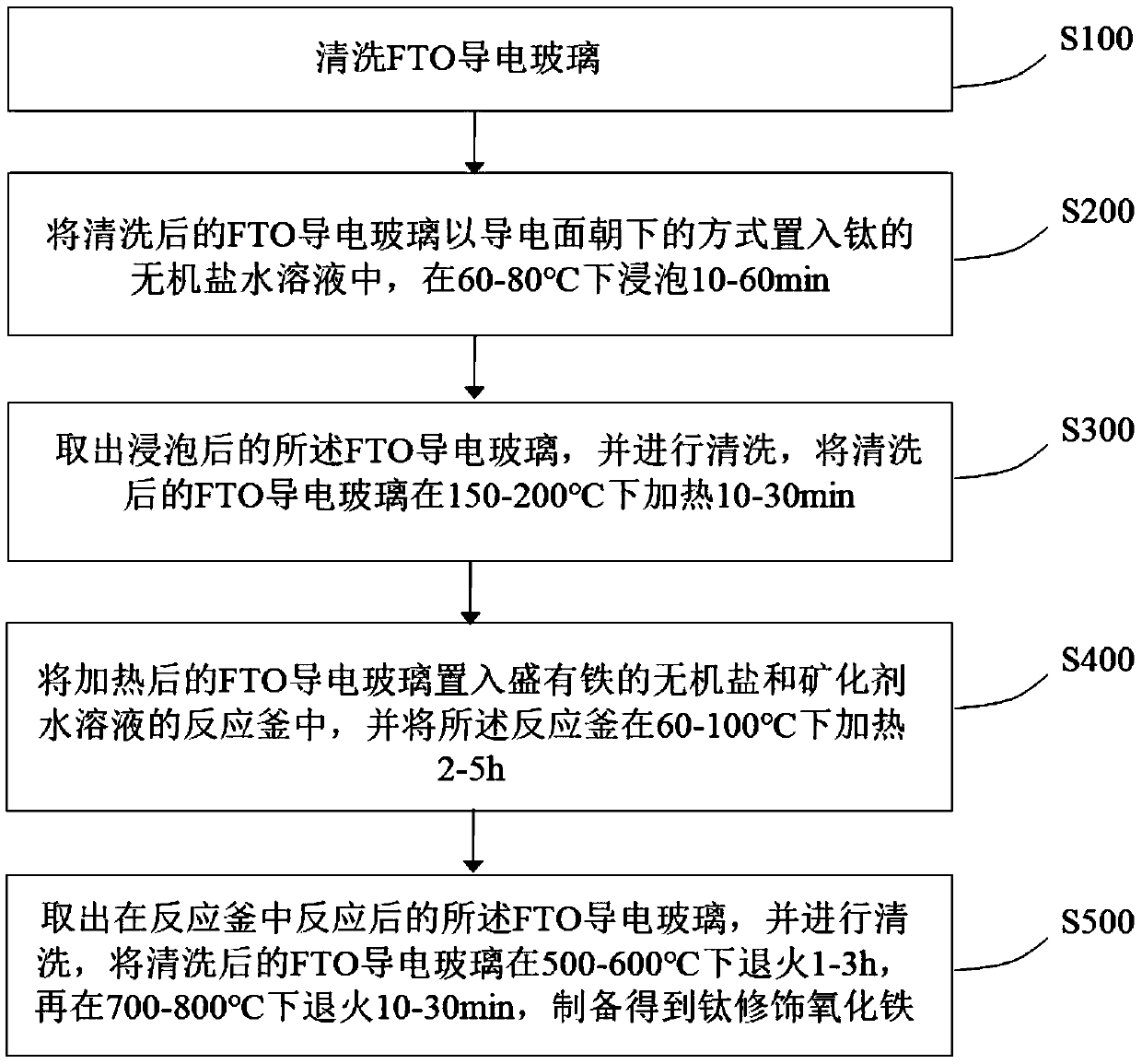
The invention provides a self-driven water photoelectrolysis system based on a friction nano power generator. The system comprises a friction nano power generator for converting external mechanical energy into electric energy, a transformer for converting high voltage electricity generated by the friction nano power generator into low voltage electricity, a rectifier for converting alternating current after transformation by the transformer into direct current, and a water photoelectrolysis device connected with the rectifier and used for generating hydrogen by the action of illumination and the direct current. The system achieves an unexpected technical effect through a relatively simple structure, and the hydrogen generation efficiency of the system is extremely high. In addition, iron oxide is modified by titanium, so that the defect of poor conductivity of iron oxide is overcome; and the efficiency of converting light energy to chemical energy is greatly improved. A method of preparing titanium modified iron oxide is simple in process and easy to operate. A selected semiconductor photocatalysis material, namely iron oxide, is high in light absorptivity and stability and low incost.
Owner:SUZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com